- Review
- Open access
- Published:
Development of therapeutic antibodies for the treatment of diseases
Journal of Biomedical Science volume 27, Article number: 1 (2020)
Abstract
It has been more than three decades since the first monoclonal antibody was approved by the United States Food and Drug Administration (US FDA) in 1986, and during this time, antibody engineering has dramatically evolved. Current antibody drugs have increasingly fewer adverse effects due to their high specificity. As a result, therapeutic antibodies have become the predominant class of new drugs developed in recent years. Over the past five years, antibodies have become the best-selling drugs in the pharmaceutical market, and in 2018, eight of the top ten bestselling drugs worldwide were biologics. The global therapeutic monoclonal antibody market was valued at approximately US$115.2 billion in 2018 and is expected to generate revenue of $150 billion by the end of 2019 and $300 billion by 2025. Thus, the market for therapeutic antibody drugs has experienced explosive growth as new drugs have been approved for treating various human diseases, including many cancers, autoimmune, metabolic and infectious diseases. As of December 2019, 79 therapeutic mAbs have been approved by the US FDA, but there is still significant growth potential. This review summarizes the latest market trends and outlines the preeminent antibody engineering technologies used in the development of therapeutic antibody drugs, such as humanization of monoclonal antibodies, phage display, the human antibody mouse, single B cell antibody technology, and affinity maturation. Finally, future applications and perspectives are also discussed.
Background
Monoclonal antibodies (mAbs) are produced by B cells and specifically target antigens. The hybridoma technique introduced by Köhler and Milstein in 1975 [1] has made it possible to obtain pure mAbs in large amounts, greatly enhancing the basic research and potential for their clinical use. Other scientific and technological advances have also enabled the successful translation of mAbs to the clinic. Around the world, at least 570 therapeutic mAbs have been studied in clinical trials by commercial companies [2], and 79 therapeutic mAbs have been approved by the United States Food and Drug Administration (US FDA) and are currently on the market [3], including 30 mAbs for the treatment of cancer (Table 1).
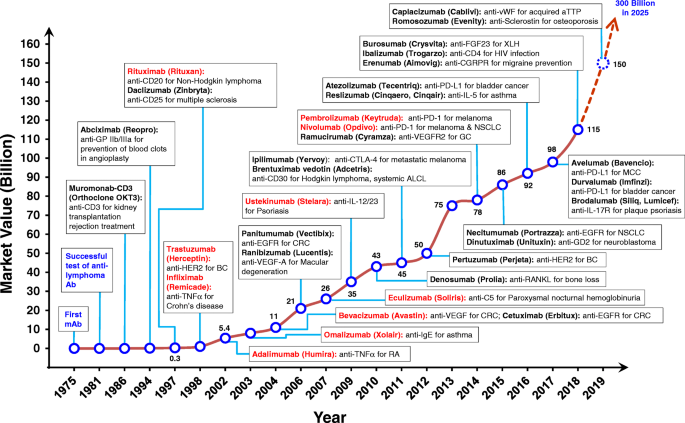
The increasing importance of therapeutic mAbs is apparent (Fig. 1), as mAbs have become the predominant treatment modality for various diseases over the past 25 years. During this time, major technological advances have made the discovery and development of mAb therapies quicker and more efficient. Since 2008, 48 new mAbs have been approved, contributing to a total global market of 61 mAbs in clinical use at the end of 2017, according to the US FDA. Strikingly, a total of 18 new antibodies were granted approval by the US FDA from 2018 to 2019 – this number was tallied from information contained on various websites, including the antibody society [3], the database of therapeutic antibodies [4], and company pipelines and press releases. A list of antibody-based drugs approved by the US FDA is shown in Table 1.
Timeline from 1975 showing the successful development of therapeutic antibodies and their applications. Many biotech companies that promised antibodies as anticancer “magic bullets” were launched from 1981 to 1986. The height of the line and numerical annotations represent the estimated market value of mAb therapeutics in each indicated year (shown as billions of US dollars). Antibodies colored in red represent the top 10 best-selling antibody drugs in 2018. Ab, antibody; ALCL, systematic anaplastic large-cell lymphoma; aTTP, acquired thrombotic thrombocytopenic purpura; BC, breast cancer; CD, cluster of differentiation; CGRP, calcitonin gene-related peptide; CGRPR, calcitonin gene-related peptide receptor; CRC, colorectal cancer; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFR, epidermal growth factor receptor; FGF, fibroblast growth factor; GC, gastric cancer; GD2, disialoganglioside GD2; HER2, human epidermal growth factor receptor 2; IgE, immunoglobulin E; IL, interleukin; IL-17R, interleukin-17 receptor; mAb, monoclonal antibody; MCC, merkel-cell carcinoma; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TNFα, tumor necrosis factor α; RA, rheumatoid arthritis; RANKL, receptor activator of nuclear factor kappa-B ligand; VEGF-A, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2; vWF, von Willebrand factor; XLH, X-linked hypophosphatemia
The first therapeutic mAb, muromonab-CD3 (Orthoclone OKT3), was approved by the US FDA in 1986 [5] and comprises a murine mAb against T cell-expressed CD3 that functions as an immunosuppressant for the treatment of acute transplant rejection. The marketing end date of muromonab-CD3 is on July 30th, 2011 (Table 1). To overcome problems of decreased immunogenic potential and efficacy, while making possible the therapeutic use of antibodies for an extended duration, researchers developed techniques to transform rodent antibodies into structures more similar to human antibodies, without loss of binding properties. The first chimeric antibody, anti-GPIIb/IIIa antigen-binding fragment (Fab) (abciximab), was approved in 1994 by the US FDA for inhibition of platelet aggregation in cardiovascular diseases (Fig. 1). The drug was developed by combining sequences of the murine variable domain with human constant region domain (Fig. 2b) [6, 7]. Then the first mAb with an oncologic indication, rituximab, a chimeric anti-CD20 IgG1 approved for non-Hodgkin’s lymphoma in 1997 by US FDA (Fig. 1) [8, 9].
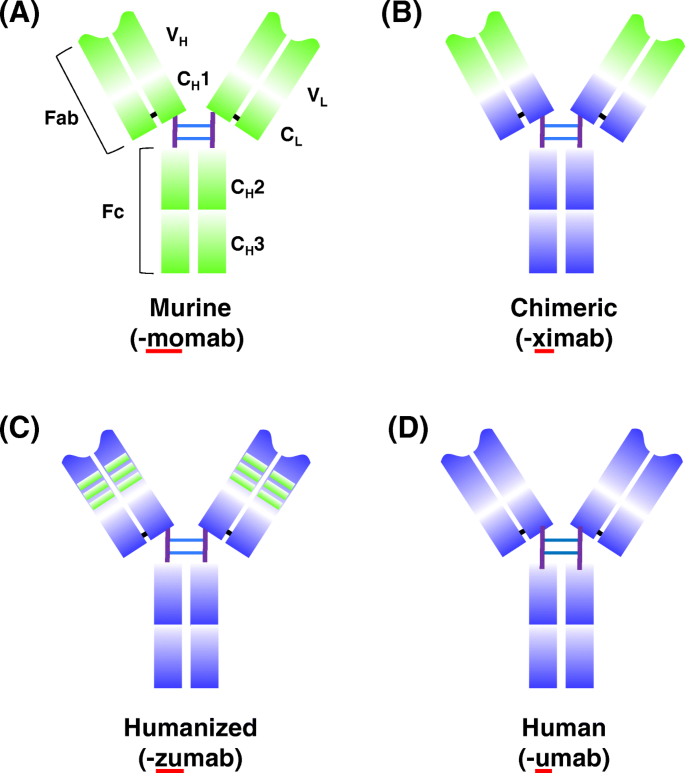
Schematic overview of antibody humanization from murine antibodies (green domains) to fully human antibodies (orange domains) and associated suffixes. a The murine monoclonal antibody. b The chimeric monoclonal antibody: variable regions are of murine origin, and the rest of the chains are of human origin. c Humanized monoclonal antibody: only includes the hypervariable segments of murine origin. d Human monoclonal. CH: domains of the constant region of the heavy chain; CL: constant domain of the light chain; Fab and Fc: fragments resulting from proteolysis; VH: variable domain of the heavy chain; VL: variable domain of the light chain
One exceptional advance that accelerated the approval of therapeutic mAbs was the generation of humanized antibodies by the complementary-determining region (CDR) grafting technique [10]. In CDR grafting, non-human antibody CDR sequences are transplanted into a human framework sequence in order to maintain target specificity [10] (Fig. 2c). The first humanized mAb approved by the US FDA in 1997 was the anti-IL-2 receptor, daclizumab, for the prevention of transplant rejection (Fig. 1) [11]. The humanization of antibodies made it possible to clinically apply a new class of biologics directed against diseases that require long-term treatment, such as cancer and autoimmune diseases [12].
Based on the success of humanized mAbs in the clinic, a key discovery technology to obtain fully human mAbs (Fig. 2d) was developed in 1990 by Sir Gregory P. Winter [10, 13]. This technique was based on phage display, wherein diverse exogenous genes are incorporated into filamentous bacteriophages to compose a library. The library proteins are then presented on the phage surface as fusions with a phage coat protein, allowing the selection of specific binders and affinity characteristics. The phage display technique was first introduced by George P. Smith [14] and comprises a powerful method for the rapid identification of peptides or antibody fragments, such as single chain fragment variable (scFv) or Fab, that bind a variety of target molecules (proteins, cell-surface glycans and receptors) [15] (Fig. 3b). The Nobel Prize in Chemistry 2018 was awarded to George P. Smith and Sir Gregory P. Winter. George Smith developed phage-displayed peptides, which can be used to evolve new proteins [14]. Gregory P. Winter was able to apply the phage-displayed antibody library to the discovery and isolation of antibodies [13]. Phage display technology has also been used for antibody maturation by site-directed mutagenesis of CDR and affinity selection. Based on these techniques, the first fully human therapeutic antibody, adalimumab (Humira), an anti-tumor necrosis factor α (TNFα) human antibody [16], was approved in 2002 by the US FDA for rheumatoid arthritis (Fig. 1). Until now, nine human antibody drugs generated by phage display have been approved by the US FDA (Table 5).
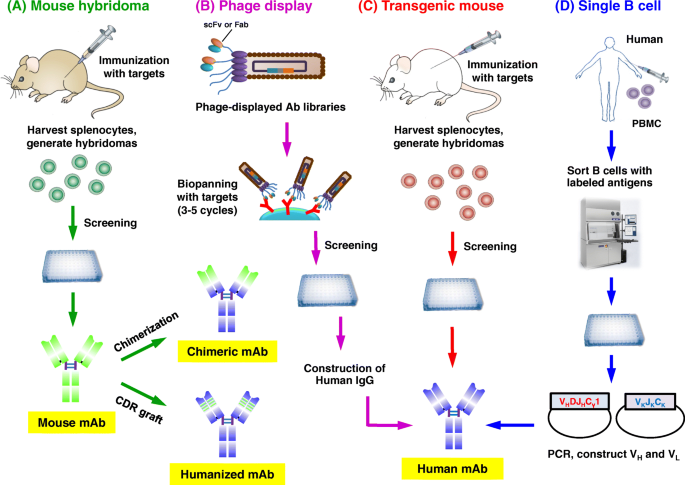
Approaches for the development of therapeutic antibodies. a The traditional mouse hybridoma technique starts by immunization of mice with desired antigens to trigger an immune response. Harvested splenocytes are fused with myeloma cells to produce hybridoma cells that persistently secrete antibodies. After the screening, selected leads are used to generate chimeric or humanized antibodies. b Phage display. A human phage-displayed human antibody library is used to select antigens of interest. After 3–5 rounds of biopanning, immuno-positive phage clones are screened by ELISA; then DNA sequences are analyzed to construct and express human IgGs. c Transgenic mouse. Similar to the mouse hybridoma technique or single B cell methods. d The single B cell technique. From infected or vaccinated donors, PBMCs are prepared for isolation of suitable B cells by flow cytometry. Following the RT-PCR, VH and VL information of each B cell informs the generation of human mAbs
Transgenic animals represent another technology for obtaining fully human mAbs (Fig. 3c). This technology was introduced in 1994 by the publication of two transgenic mouse lines, the HuMabMouse [35] and the XenoMouse [36]. The lines were genetically modified such that human immunoglobulin (Ig) genes were inserted into the genome, replacing the endogenous Ig genes and making these animals capable of synthesizing fully human antibodies upon immunization [35, 37]. The first human antibody generated in a transgenic mouse to anti-epidermal growth factor receptor (EGFR), panitumumab, was approved by the US FDA in 2006 (Fig. 1) [38, 39]. The number of fully human antibodies made from transgenic mice has increased rapidly, with the number of approved drugs currently at 19 (Table 5). Depending on the immunization protocol, high-affinity human antibodies can be obtained through further selection of hybridoma clones generated from immunized transgenic mice. Using a theoretically similar approach, the generation of neutralizing human antibodies from human B cells has also yielded promising results for infectious disease therapeutics.
The recent development of bispecific antibodies offers attractive new opportunities for the design of novel protein therapeutics. A bispecific antibody can be generated by utilizing protein engineering techniques to link two antigen binding domains (such as Fabs or scFvs), allowing a single antibody to simultaneously bind different antigens. Thus, bispecific antibodies may be engineered to exhibit novel functions, which do not exist in mixtures of the two parental antibodies. Most bispecific antibodies are designed to recruit cytotoxic effector cells of the immune system to target pathogenic cells [40]. The first approved bispecific antibody was catumaxomab in Europe in 2009 [41]. Catumaxomab targets CD3 and EpCAM to treat solid tumors in patients with malignant ascites. However, this drug was withdrawn from the market in 2017 for commercial reasons. Currently, two bispecific antibodies have obtained US FDA approval and are on the market. First, blinatumomab is a bispecific T-cell engager (BiTE) that targets CD3 and CD19 for treatment of B-cell precursor acute lymphoblastic leukemia (ALL) [42]. Second, emicizumab is a full-size bispecific IgG with natural architecture, which binds to activated coagulation factors IX and X for the treatment of haemophilia A [43]. To date, there are more than 85 bispecific antibodies in clinical trials, about 86% of which are under evaluation as cancer therapies [40]. The concepts and platforms driving the development of bispecific antibodies continue to advance rapidly, creating many new opportunities to make major therapeutic breakthroughs.
While mAbs are routinely used in biochemistry, molecular and cellular biology, and medical research, perhaps the most beneficial application is their use as therapeutic drugs for the treatment of human diseases, such as cancer, asthma, arthritis, psoriasis, Crohn’s disease, transplant rejection, migraine headaches and infectious diseases (Table 1). Important advances in antibody engineering made over the past decade have enhanced the safety and efficacy of the therapeutic antibodies. These developments, along with a greater understanding of the immunomodulatory properties of antibodies, have paved the way for the next generation of new and improved antibody-based drugs for the treatment of human diseases.
Clinical applications and market for therapeutic antibodies
Therapeutic antibodies currently approved as disease treatments
The mAb market enjoys a healthy pipeline and is expected to grow at an increasing pace, with a current valuation of $115.2 billion in 2018 [44]. Despite this high growth potential, new companies are unlikely to take over large shares of the market, which is currently dominated by seven companies: Genentech (30.8%), Abbvie (20.0%), Johnson & Johnson (13.6%), Bristol-Myers Squibb (6.5%), Merck Sharp & Dohme (5.6%), Novartis (5.5%), Amgen (4.9%), with other companies comprising the remaining 13% [44].
Many mAbs products achieved annual sales of over US$3 billion in 2018 (Fig. 1), while six (adalimumab, nivolumab, pembrolizumab, trastuzumab, bevacizumab, rituximab) had sales of more than $6 billion (Table 2). Adalimumab (Humira) had the highest sales figure ever recorded for a biopharmaceutical product, nearly $19.9 billion. The top ten selling mAb products in 2018 are listed in Table 2. Top-selling mAb drugs were ranked based on sales or revenue reported by biological or pharmacological companies in press announcements, conference calls, annual reports or investor materials throughout 2018. For each drug, the name, sponsors, disease indications, and 2018 sales are shown.
mAbs are increasingly used for a broad range of targets; oncology, immunology, and hematology remain the most prevalent medical applications [45]. Most mAbs have multiple disease indications and at least one that is cancer-related (lymphoma, myeloma, melanoma, glioblastoma, neuroblastoma, sarcoma, colorectal, lung, breast, ovarian, head and neck cancers). As such, oncological diseases are the medical specialty most accessible to mAb treatments [45]. Moreover, the number of target proteins known to function as either stimulatory or inhibitory checkpoints of the immune system has dramatically expanded, and numerous antibody therapeutics targeting programmed cell death protein 1 (PD-1, cemiplimab, nivolumab, pembrolizumab), its ligand programmed death-ligand 1 (PD-L1, durvalumab, avelumab, atezolizumab) or cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4, ipilimumab) have been granted marketing approvals [46].
Adalimumab (Humira) was the world’s best-selling drug in 2018. Adalimumab is a subcutaneously administered biological disease modifier used for the treatment of rheumatoid arthritis and other TNFα-mediated chronic debilitating diseases. It was originally launched by Abbvie in the United States after gaining approval from the US FDA in 2002. It has been shown that Adalimumab reduces the signs and symptoms of moderate to severe rheumatoid arthritis in adults, and it is also used to treat psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, psoriasis, hidradenitis suppurativa, uveitis, and juvenile idiopathic arthritis [47, 48]. It may be used alone or in combination with disease-modifying anti-rheumatic drugs [49].
Immune checkpoints are important for maintaining self-tolerance and tempering physiologic immune responses in peripheral tissues. Therefore, the molecules underlying checkpoints have recently drawn considerable interest in cancer immunotherapy [50]. Both nivolumab (Opdivo) and pembrolizumab (Keytruda) are anti-PD-1 mAbs and were the second and third best-selling mAb drugs in 2018 (Table 2). Nivolumab is a human antibody, which blocks a signal that normally prevents activated T cells from attacking cancer cells. The target for nivolumab is the PD-1 receptor, and the antibody blocks the interaction of PD-1 with its ligands, PD-L1 and PD-L2, releasing PD-1 pathway-mediated immune inhibition [51, 52]. Pembrolizumab is a humanized antibody used in cancer immunotherapy to treat melanoma, lung cancer, head and neck cancer, Hodgkin’s lymphoma, and stomach cancer [53,54,55]. Pembrolizumab is a first-line treatment for NSCLC if cancer cells overexpresse PD-L1 and have no mutations in EGFR or in anaplastic lymphoma kinase [56, 57]. Large randomized clinical trials indicated that NSCLC patients treated with nivolumab and pembrolizumab (both approved by the US FDA in 2014) showed increased overall survival compared with docetaxel, the standard second-line treatment [58].
A total of 12 new mAbs were approved in the US during 2018. The majority of these products were approved for non-cancer indications, perhaps reflecting the higher approval success rate for antibodies as treatments for other diseases. Three antibodies (erenumab, galcanezumab, and fremaezumab) were approved for migraine prevention, and one (Ibalizumab) is used for human immunodeficiency virus (HIV) infection. The three migraine-preventing drugs, Erenumab (Aimovig), galcanezumab (Emgality), and fremaezumab (Ajovy), are mAbs that block the activity of calcitonin gene-related peptide (CGRP) receptor in migraine etiology [59]. CGRP acts through a heteromeric receptor, which is composed of a G protein-coupled receptor(calcitonin receptor-like receptor: CALCRL) and receptor activity-modifying protein 1 (RAMP1) [60, 61]. Both galcanezumab and fremaezumab bind to CGRP and block its binding to the receptor. However, erenumab is the only one of the three antibodies to target the extracellular domains of human G protein-coupled receptors CALCRL and RAMP1,interfering with the CGRP binding pocket [62].
Many mAbs are under development for treatment of infectious diseases, currently only four have been approved by the US FDA: raxibacumab and obiltoxaximab for treatment of inhalational anthrax [63], palivizumab for prevention of respiratory syncytial virus in high-risk infants [64], and ibalizumab for treatment of HIV infection patients [65]. Ibalizumab (Trogarzo) is a humanized IgG4 mAb that is used as a CD4 domain 2-directed post-attachment HIV-1 inhibitor. The US FDA approved ibalizumab for adult patients infected with HIV who were previously treated and are resistant to currently available therapies.
Therapeutic antibodies currently in clinical trials
Companies are currently sponsoring clinical studies for more than 570 mAbs. Of these, approximately 90% are early-stage studies designed to assess safety (Phase I) or safety and preliminary efficacy (Phase I/II or Phase II) in patient populations. Most of the mAbs in Phase I (~ 70%) are for cancer treatment, and the proportions of mAbs intended to treat cancer are similar for those currently in Phase II and late-stage clinical studies (pivotal Phase II, Phase II/III or Phase III) [2].
Twenty-nine novel antibody therapeutics were in late-stage clinical studies for non-cancer indications in 2018. Among the trials for these mAbs, no single therapeutic area predominated, but 40% were for immune-mediated disorders, which comprised the largest group. From this group of potential treatments, leronlimab and brolucizumab entered regulatory review by the end of 2018, and five mAbs (eptinezumab, teprotumumab, crizanlizumab, satralizumab, and tanezumab) may enter regulatory review in 2019. In comparison, there were 33 novel antibody therapeutics in late-stage clinical studies for cancer indications in 2018. Antibody therapeutics for solid tumors clearly predominated, with less than 20% of the candidates intended solely for hematological malignancies. Five mAbs (isatuximab, spartalizumab, tafasitamab, dostarlimab, and ublituximab) license applications were submitted to the US FDA in 2019 [2].
Isatuximab is an anti-CD38 IgG1 chimeric mAb under evaluation as a treatment for patients with multiple myeloma (MM). Combinations of isatuximab and different chemotherapies are being tested in three Phase III studies (ICARIA, IKEMA, and IMROZ) on MM patients. The ICARIA study (NCT02990338) is evaluating the effects of isatuximab in combination with pomalidomide and dexamethasone compared to chemotherapy only in patients with refractory or relapsed MM. Pivotal Phase III ICARIA-MM trial results demonstrated that isatuximab combination therapy showed statistically significant improvements compared to pomalidomide and dexamethasone alone in patients with relapsed or refractory MM in 2019. The US FDA has accepted for review the biologics license application for isatuximab for the treatment relapsed or refractory MM patients. The target action date for the FDA decision is April 2020 [66]. The IKEMA (NCT03275285) and IMROZ (NCT03319667) studies are evaluating the isatuximab with other chemotherapeautic combinations in MM patients [67].
Spartalizumab is a humanized IgG4 mAb that binds PD-1 with sub-nanomolar affinity and blocks its interaction with PD-L1/PD-L2, preventing PD-1-mediated inhibitory signaling and leading to T-cell activation. Clinical study of Spartalizumab is underway with a randomized, double-blind, placebo-controlled Phase III COMBI-i study (NCT02967692), which is evaluating the safety and efficacy of dabrafenib and trametinib in combination with spartalizumab compared to matching placebo in previously untreated patients with BRAF V600-mutant unresectable or metastatic melanoma. The primary endpoints of the study are an assessment of dose-limiting toxicities, changes in PD-L1 levels and CD8+ cells in the tumor microenvironment, and progression-free survival. Key secondary endpoints are overall survival, overall response rate and duration of response. The estimated primary completion date of the study is September 2019 [68].
Dostarlimab is an anti-PD-1 mAb that may be useful as a treatment for several types of cancers. GlaxoSmithKline announced results from a Phase I dose escalation and cohort expansion study (GARNET; NCT02715284) in 2018, which is expected to support a biologics license application submission to the US FDA in 2019. Dostarlimab is being assessed in patients with advanced solid tumors who have limited available treatment options in the GARNET study. The drug is administered at a dose of 500 mg every 3 weeks for the first 4 cycles, and 1000 mg every 6 weeks thereafter in four patient cohorts: microsatellite instability high (MSI-H) endometrial cancer, MSI-H non-endometrial cancer, microsatellite-stable endometrial cancer, and non-small cell lung cancer. Dostarlimab is also being evaluated in another Phase III study (NCT03602859), which is comparing platinum-based therapy with dostarlimab and niraparib versus standard of care platinum-based therapy as first-line treatment of Stage III or IV non-mucinous epithelial ovarian cancer [69].
Ublituximab is a glyco-engineered anti-CD20 antibody currently under clinical investigation in five late-stage clinical studies for different cancers (chronic lymphocytic leukemia, CLL, non-Hodgkin’s lymphoma) and non-cancer (multiple sclerosis) indications. Three Phase III studies are exploring the efficacy of ublituximab in combination with other anti-cancer agents. Among these studies, the UNITY-CLL Phase III study (NCT02612311) is evaluating the combination of ublituximab and TGR-1202, a PI3K delta inhibitor, compared to anti-CD20 obinutuzumab plus chlorambucil in untreated and previously treated CLL patients. Two other Phase III studies (ULTIMATE 1, NCT03277261 and ULTIMATE 2, NCT03277248) are evaluating the efficacy and safety of ublituximab compared to teriflunomide in 440 patients with relapsing multiple sclerosis [70].
Methodologies for developing therapeutic antibodies
Human, humanized, chimeric, and murine antibodies respectively account for 51, 34.7, 12.5, and 2.8% of all mAbs in clinical use, making human and humanized mAbs the dominant modalities in the field of therapeutic antibodies. In the next section, we first introduce techniques for antibody humanization. Then, we describe three technical platforms related to the generation of fully human antibodies, including phage display, transgenic mice and single B cell antibody isolation (Fig. 3). Last, we describe the use of an affinity maturation method to optimize antibody binding activity.
Humanization of mAbs
Due to the availability, low cost and quick production time for mouse mAbs, humanization of mouse mAbs has been implemented on a large scale. Non-humanized murine mAbs have many disadvantages as treatments. For example, patients treated with mouse mAbs will produce a rapid human anti-mouse antibody (HAMA) response. HAMAs will not only hasten the clearance of mouse mAbs but may also produce undesirable allergic reactions and tumor penetration. Moreover, the ability of patients to initiate antibody-dependent cellular cytotoxicity (ADCC) in response to murine fragment crystallizable region (Fc) is limited. On the other hand, humanized mAbs are able to effectively exert effector functions while decreasing the immunogenicity of murine antibodies.
Generation of humanized mAbs
Humanized mAbs, of which only the CDRs of the light and heavy chains are murine, entered clinical development for the first time in 1988 [71, 72]. CDR grafting is one of the most popular techniques in the production of humanized mAbs and was originally developed by Gregory P. Winter in 1986 [9]. Using this technology, non-human CDR sequences are transplanted into human framework sequences, allowing the antibody to maintain the binding activity to the target antigen [9]. The first US FDA approved CDR-grafted humanized mAb occurred in 1997 for daclizumab, which binds the IL-2 receptor and is used to prevent transplant rejection [11]. Queen and collaborators [73] developed daclizumab not only using CDR grafting, but also using the human framework that is maximally homologous to the murine framework, in order to decrease the loss of antigen recognition. In some cases, certain amino acids in the murine framework are crucial to maintain antibody binding activity. These residues may cooperate with CDRs to present an antibody paratope or directly interact with antigens. Currently, these crucial framework residues can be identified by observing the structure of antibody-antigen complex by X-ray crystallography, cryo-electron microscopy and computer-aided protein homology modelling [74]. The positions of amino acids in the framework may then be considered for restore by ‘human back to mouse’ mutations in CDR-grafted humanized antibodies, thereby improving the affinity and stability of the final product. Currently, web servers are being developed by integrated bioinformatics and antibody structure databases for rendering humanization experiments [75, 76]. They provide the tools for human template selection, grafting, back-mutation evaluation, and antibody modeling. However, if the binding activity of antibodies is still compromised, it should be further performed affinity maturation to improve this situation.
Multiple methods have been developed to quantify the humanness of the variable region of mAbs. Abhinandan and Martin designed a tool called “H-score” to assess the “degree of humanness” of antibody sequences, which calculates the mean sequence identity compared to a subset of human variable region sequences database [77]. A germinality index was defined subsequent to assist germline humanization of a macaque antibody [78]. G-score was derived from the H-score to improve classification of germline framework sequence [79]. T20 score analyzer was established under a large database of ~ 38,700 human antibody variable region sequences to clearly separate human sequences from mouse sequences and many other species as well [80]. It was used to reveal similarities between humanized antibodies and fully human antibodies. These humanness score tools are available online and allow assisting the generation of humanized antibody [80].
The use of humanized antibodies has helped greatly to improve clinical tolerance of mAb therapeutics. Such intricate control over antibody sequences has opened the door to engineering mAbs for a wide range of possible applications in medicine. Currently, half of all mAbs used to treat humans are chimeric or humanized (Fig. 2, Table 1). One of the most well-known humanized antibodies is Trastuzumab (Herceptin), which was approved in 1998 and achieved annual sales of over $7 billion in 2018 (Table 2). Trastuzumab is used for the treatment of patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer and gastroesophageal junction adenocarcinoma [57, 58].
Immunogenicity of antibody-based therapeutics
The use of mAbs in a clinical setting should have several essential biophysical properties, including high antigen binding activity, high stability, and low immunogenicity [81]. Antibody immunogenicity means the degree of the host immune system can recognize and react to these therapeutic agents. Anti-drug antibodies (ADA) induced by the immune system can be found while immunogenicity occurring in patients administered with antibody drugs. Anti-drug antibodies have the potential to neutralize therapeutic agents, which can reduce the efficacy of the drugs [82]. Importantly, anti-drug antibodies may further cause adverse effects ranging from skin rashes to systemic inflammatory responses in the patients, which can impact both safety and efficacy of the antibody drugs in clinic use [83]. Immunogenicity is influenced by several factors, such as drug dosage, administration strategy (route and combination), impurities contamination, aggregates arising from Ab/Ag binding complex, and structural features (sequence variation and glycosylation) [84].
Humanized antibodies harbor human sequence in constant regions and nearly all human sequence in Fv, of which only CDRs are murine grafted. Antibodies of more human-like usually allow them to be higher tolerant and lower immunogenic in a clinical setting. For example, Perpetua et al. showed a case to support this concept [85]. They compared a humanized anti-CD52 antibody with its parental murine version and demonstrated humanization offers a significant reduction in immunogenicity. However, humanized antibodies retain murine CDRs which could be regarded as foreign antigens by host immune systems and eventually arise immunogenicity. For example, ADA was detected in 0.5% of women with metastatic breast cancer, who were treated with Trastuzumab during their therapeutic courses [86]. Recently, an immunogenicity analysis result from clinical data showed the ADA rates were 7.1% (21/296) in the HER-2 positive breast cancer patients with treatment of Trastuzumab [87]. The variation of immunogenicity in the same antibody drug may be caused by many potential factors: the age, race, genetic background, other related diseases, and programs of drugs administration.
The CDRs and frameworks of fully human antibodies are derived for human immunoglobulin gene repertoires, thus which can theoretically bypass immunogenicity. However, several fully human antibodies have been reported to induce marked immune responses when administrated in patients [88]. Adalimumab (Humira), a human IgG1, has been reported to generate significant immune responses through eliciting anti-idiotypic antibody in a part of patients (5–89%) which varies depending on the disease and the therapy [89, 90]. Golimumab (Simponi), a fully human anti-TNFα antibody, combining with methotrexate for treatment of rheumatoid arthritis cause 16% of patients producing anti-drug antibodies [91]. One reason of these scenarios is that Fv sequence of human antibodies is not identical to human germline: antibody evolution through VJ and VDJ random recombination, as well as affinity maturation naturally occurring in vivo through somatic hypermutation. Until now, there are no in vitro or in-silico assays can precisely analyze the immunogenicity of antibody. In vivo assessments are usually used to evaluate the immunogenicity, of which the result will ameliorate design and engineering of antibody therapeutics to reduce the potential for inducing anti-drug antibodies.
Generation of human antibodies by phage display
Overview of antibody phage libraries
Phage display is the first and still the most widely used technology for in vitro antibody selection. The strategy was developed based on the excellent work of George P. Smith in 1985 [14], who used recombinant DNA techniques to fuse foreign peptides with a coat protein (pIII) of bacteriophage M13 in order to display peptides on the bacteriophage surface. He then created “antibody-selectable phage vectors” and described an in vitro method that enabled affinity selection of antigen-specific phage-displayed antibodies from 108-fold excess phage pools [92]. It was later discovered that scFv, small antibody formats, can be expressed on phage filaments. At the time, there were three different research institutions independently establishing phage-displayed scFv or Fab antibody libraries: the MRC Laboratory of Molecular Biology in the UK [13, 93, 94], the German Cancer Research Center in Germany [95], and Scripps Research Institute in the USA [96]. Since then, these phage-displayed antibody libraries have proven to be a reliable discovery platform for the identification of potent, fully human mAbs [97].
The process of identifying mAbs from a phage-displayed library begins with antibody-library construction (Fig. 4a). The variable heavy (VH) and variable light (VL) polymerase chain reaction (PCR) products, representing the Ig gene-encoding repertoire, are ligated into a phage display vector (phagemid). High quality mRNA from human peripheral blood mononuclear cells (PBMCs) is reverse-transcribed into cDNA. The different VH and VL chain-region gene families are then amplified using specific primers to amplify all transcribed variable regions within the Ig repertoire [98, 99]. The format of antibodies in a phage-displayed library can be either scFv or Fab fragments (Fig. 4b); scFvs are composed of the VH and VL domain connected by a short flexible linker. Antibody Fab fragments displayed on the phage coat protein have comparably higher structural stability and can be readily converted to intact IgG antibodies, usually without impairing binding activity [100, 101]. The elegance of phage-displayed libraries is apparent in the linkage between antibody phenotype (specificity and sensitivity) and genotype (genetic information) via the phage particle. Due to the small size and high solubility (1013 particles/ml) of phage particles, repertoire sizes up to 1011 independent clones can be efficiently produced and displayed in a single library [102,103,104].
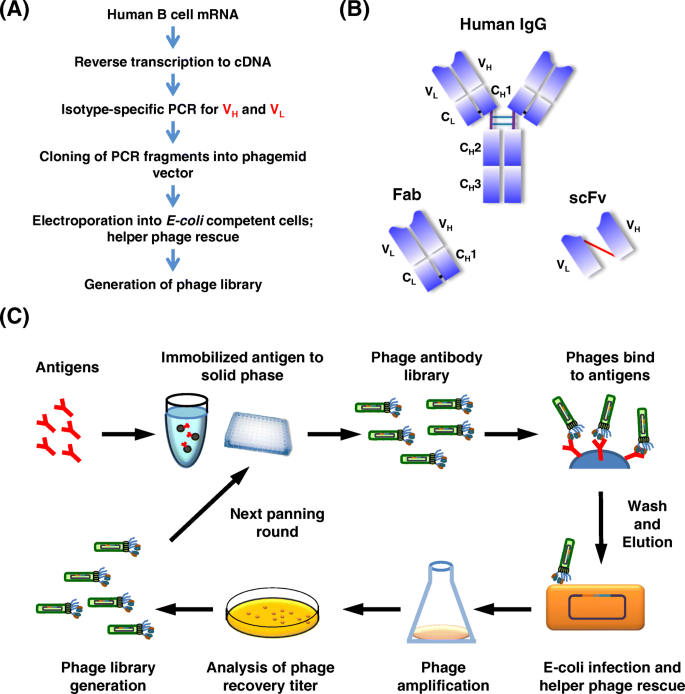
Construction and affinity selection with phage-display antibody library. a Outline of the procedure for constructing a phage-displayed antibody (Fab or scFv) library. b Structure of IgG molecule. Fab consists of the light chain and the first two domains of the heavy chain. scFv is composed of the variable heavy (VH) and variable light (VL) domains joined by a short flexible polypeptide linker. c Biopanning with a phage-displayed library. Initial pools of antibodies on the surface of phages are applied to antigens immobilized on a solid surface, e.g., ELISA plates or magnetic beads. Non-specific phages are removed by stringent washing. Antigen-bound phages are eluted and re-infected into E. coli to produce a subset of phages for the next cycle of panning. After several rounds, the antigen-binding clones are sufficiently enriched and individual clones can be selected for further analysis
Gene repertoires for phage display libraries can be obtained from naïve or immunized animals, or the libraries may be synthetically constructed using randomized CDR sequences within fixed frameworks. Phage display naïve antibody libraries are constructed from rearranged V genes of IgM repertoires. Because the gene sequences are derived from B cells of human donors, the naïve libraries are relatively close to the human antibody germ line and have a low risk of immunogenicity. The main advantage of an immunized library over a naïve library is that antibody genes in the immunized library have undergone natural affinity maturation in vivo, allowing the development of high-affinity antibodies against the target. However, this approach requires that immunogenic response can be successfully induced by the antigen of interest, and new libraries must be prepared for each new target. Single large naïve [94, 104, 105] and synthetic [102, 106, 107] libraries have yielded high affinity antibodies (sub-nanomolar range) against a wide spectrum of targets. Therefore, such non-immunized libraries have the distinct advantages of avoiding issues with immunological tolerance in immunized mice, and they do not require new immunized libraries for each new target.
Currently, almost all widely accessible commercial libraries are based on highly diverse non-immunized gene repertoires, which allow selection of antibodies against a virtually unlimited number of targets [108]. It is worth noting that most antibody drugs that have undergone evaluation in clinical trials originated from a few company-owned libraries. These libraries include: Cambridge Antibody Technology’s (now MedImmune, a subsidiary of AstraZeneca) scFv-fragment library, Dyax Corp’s (now Shire) human Fab-fragment libraries, scFv and Fab libraries from XOMA, and the fully synthetic human combinatorial antibody scFv (HuCAL) and Fab (HuCALGold) libraries developed by MorphoSys [97].
Affinity selection of human antibodies
Antibody libraries are typically screened by iterative selection cycles to enrich target-binding phages, followed by amplification of the bound phages in E. coli cells. The affinity screening process for antibody libraries is called biopanning (Fig. 4c). Repeated rounds of selection allow for the enrichment of very rare antigen-binding phage clones, eventually resulting in the selection of the most highly specific binders. This stringent process is a critical feature of phage display that allows mAbs to be isolated in a period as short as a couple of weeks, far more quickly than the traditional hybridoma method [99, 109]. For in vitro selection, it is necessary to immobilize target antigens on a solid surface. Polystyrene surfaces with high protein binding capacity, such as 96-well immuno-plates and immuno-tubes, are widely used for antigen immobilization. Additionally, magnetic beads with protein G/A, streptavidin, maleimide or N-hydroxysuccinimide can be used to immobilize antigens and perform biopanning in solution.
The biopanning method is not only restricted to known recombinant proteins. In fact, the phage display technique may also be utilized to select antibodies against whole cells, unveiling previously unknown antigens on the tumor cell surface [110]. Cancer is an extremely heterogeneous disease, and only a few tumor cells with stem-like properties are able to initiate and sustain tumor development; these cells are often referred as tumor initiating cells or cancer stem cells (CSCs) [111]. Phage display technology is well suited for applications in CSC research, and several antibodies have already been identified from phage display libraries for their ability to bind known CSC markers, such as CD133 and CD44 [112, 113]. Moreover, novel CSC surface markers may be identified by selecting phage-displayed antibodies that bind to a CSC-like population and then identifying the corresponding target antigens [114, 115]. The use of tumor biopsy tissue as a biological material allows researchers to probe the tumor microenvironment, which may be highly relevant for clinical use. Phage display technology has been used to probe cancer tissue biopsies in order to generate antibody fragments that specifically recognize tumor subpopulations, such as CSCs and tumor-associated endothelial cells [116,117,118] as well as other clinically relevant tumor antigens [119].
Antibodies or antibody fragments have been referred to as targeted drug delivery “missiles” for their ability to direct homing of drugs to tumors [120]. For example, immunoliposomes have been demonstrated to provide conventional liposomal drugs with cancer targeting ability, which can increase the therapeutic efficacy of anticancer drugs [121]. Cellular internalization of the targeting ligand is an essential outcome for successful tumor-targeted liposomal drug delivery [99, 122]. For this reason, an efficient phage display-based selection approach was designed to map tumor internalizing epitopes, wherein a phage-displayed library was incubated with living cancer cells at 37 °C [123]. This method was successfully applied to rapidly identify several scFvs with high rates of internalization in several types of tumors; the target antigens were subsequently identified, and intracellular drug delivery systems were further developed [99, 124].
The identification of mAbs with phage display is an entirely in vitro process. Thus, it is not restricted by immunological tolerance, allowing for the identification of antibodies against poorly immunogenic antigens or those that are difficult to obtain using animal immunization methods (e.g., glycans or toxic agents). The in vitro nature of the assay can be especially useful when identifying specific antibodies against novel or gene-mutated pathogens in an outbreak of emergent infectious diseases [125,126,127]. The antigens on pathogens usually induce a strong immune response in patients, making it common for infected individuals to naturally produce high-affinity antibodies [128]. To obtain these antibodies, mRNA from the PBMCs of pathogen-infected people can be quickly collected and used as a gene repertoire for a phage-displayed library [129]. Such a library can allow for the rapid identification of high-affinity antibodies that may then be used as guides for vaccine design, or to develop therapeutic drugs and diagnostic reagents.
Moreover, the biopanning approach has been modified to isolate and identify a few antibodies with broad neutralizing activity against pandemic influenza virus [130, 131]. Chen et al. reported the establishment of a phage-displayed Fab library derived from the PBMCs of convalescent patients infected with a novel influenza A virus H7N9, which broke out in 2013. Using this library, antibodies targeting purified H7N9 virions were isolated [132]. Two human antibodies were found to exhibit high neutralizing activity against live H7N9 virus due to their interactions with the receptor-binding site of viral hemagglutinin antigens [132, 133].
The newly emergent Middle East respiratory syndrome coronavirus (MERS-CoV) induces a severe acute respiratory syndrome-like disease with an approximately 43% mortality rate [134]. To date, no vaccines or antiviral medications are available for the prevention or clinical treatment of MERS. A large phage-displayed human naive scFv library (Mehta I/II) with 2.7 × 1010 clones from the Dana-Farber Cancer Institute was used as a resource for the isolation of human antibodies against MERS-CoV [135]. In another project, a research group in Malaysia improved panning strategies with a naïve human scFv library (library size of 109) to successfully identify mAbs specific to the MERS-CoV nucleoprotein [136].
Studies such as those described offer insights into the human antibody response to viral pathogen infection and provide examples of how the outbreak scene may be utilized to develop human antibody-based immunotherapies for the prevention and early treatment of viral pathogens [137].
The successful development of antibody drugs from phage display
Fully human therapeutic antibodies in current clinical use were discovered from either phage display or transgenic mice approaches [138]. Phage display has the advantage of allowing researchers to tailor critical characteristics of successful antibody drugs (e.g., affinity, specificity, cross-reactivity and stability). There are nine phage display-derived human antibodies currently approved by the US FDA for the treatment of human disease (Table 1), demonstrating the reliability of this technique as a platform for antibody discovery.
Adalimumab (Humira) was developed by BASF Bioresearch Corporation and Cambridge Antibody Technology. It was not only the first phage display-derived antibody granted a marketing approval, but adalimubab was also the first approved (2002) fully human mAb drug [139]. Adalimumab binds and suppresses TNFα and is approved to treat inflammatory diseases, such as rheumatoid and psoriatic arthritis, Crohn’s disease, and psoriasis. Adalimumab is the world’s best-selling drug [140] with sales of $19.9 billion in 2018 reported by AbbVie (Table 2). Cambridge Antibody Technology also identified human antibodies targeting BLYS (B lymphocyte stimulator) from phage display [141]. BLYS, a member of the tumor necrosis factor superfamily of cytokines, induces B cell proliferation and differentiation that positively correlate with systemic lupus erythematosus (SLE). This anti-BLYS antibody was named belimumab and marketed as Benlysta by GlaxoSmithKline, becoming the first drug approved (2011) for the treatment of SLE [142]. Founded in 1989, Cambridge Antibody Technology was acquired by AstraZeneca for $1.32 billion in 2006 [143].
Tyrosine kinase receptors, including EGFR and vascular endothelial growth factor receptor 2 (VEGFR2), play crucial roles in tumorigenesis, with higher expression and activation in tumors than in normal tissues. These characteristics make the receptors potentially valuable targets for drug development. Necitumumab (Portrazza) is an anti-EGFR human antibody that was identified by screening high EGFR-expressing epidermal carcinoma cells (A431) with a non-immunized phage Fab library of 3.7 × 1010 clones [105]. Necitumumab was approved in 2015 and is now a first-line therapy in combination with gemcitabine and cisplatin for the treatment of squamous NSCLC [144]. VEGFR2 is not only highly expressed in tumor endothelial cells, where it regulates tumor angiogenesis, but it also expressed on the surface of cancer cells. The anti-VEGFR2 human antibody, ramucirumab (Cyramza), was approved for the treatment of gastric cancer, metastatic NSCLC and metastatic colorectal cancer [145, 146]. The development of ramucirumab was initiated by using a phage-displayed human naïve Fab library (Dyax) containing 3.7 × 1010 independent clones for biopanning against the extracellular domain of human VEGFR2 protein [105]. Three Fab clones, D2C6, D2H2, and D1H4, were selected based on their specific binding to VEGFR2 with nanomolar affinity and their ability to neutralize VEGF-A-activated VEGFR2 signaling. Interestingly, these three Fab clones do not cross-react with murine VEGFR2 and share an identical VH sequence [147]. After affinity maturation with stringent biopanning rounds, Fab clone 1121 (IMC-1121B) was selected and showed more than the 30-fold improvement of VEGFR2-binding activity. This clone was subsequently engineered into the human intact IgG1 version (ramucirumab), which has an affinity of 50 pM [148].
PD-L1, a cell-surface protein, binds to its receptor PD-1 on immune cells, downregulating T cell inflammatory activity to promote self-tolerance by the immune system. Many types of tumors have been found to express PD-L1 on the surface of cancer cells, using the immune-suppressing action to evade immune attacks. Avelumab (Bavencio) is fully human IgG1 lamda antibody against PD-L1, which was derived from a phage-displayed naïve Fab library (Dyax) [149]. Avelumab not only blocks PD-L1 binding to PD-1, but it also induces ADCC in cancers [150]. The later function differs from other immune checkpoint-blocking antibodies. The US FDA approved avelumab in 2017 for the treatment of urothelial carcinoma and Merkel-cell carcinoma, an aggressive type of skin cancer [151].
Psoriasis is a chronic autoimmune inflammatory disorder that causes skin cell overproduction and is characterized by raised, inflamed, red lesions and plaques that are accompanied by physical pain and itching. Guselkumab (Tremfya) is a fully human antibody developed by Janssen that neutralizes anti-IL-23. The HuCAL antibody library was used to generate guselkumab under a license from MorphoSys [152, 153]. In 2017, guselkumab was granted marketing approval by the US FDA for the treatment of plaque psoriasis [154].
Hereditary angioedema is a rare disease that results in spontaneous, recurrent, and potentially life-threatening attacks of swelling in various parts of the body [155]. The disease is commonly associated with deficiency or dysfunction of C1-esterase-inhibitor and with excessive bradykinin production caused by overactive plasma kallikrein [156]. Lanadelumab (Takhzyro) is a fully human mAb derived from the Dyax phage library; it directly binds the active site of plasma kallikrein to inhibit bradykinin production. Lanadelumab was approved in 2018 in the USA and Canada for prophylaxis against attacks of hereditary angioedema in patients aged ≥ 12 years [157].
The success of a drug development effort is highly dependent on obtaining patent protection for products and technologies while avoiding infringement on patents issued to others. Therefore, intellectual property rights for phage-display antibody discovery platforms comprise a changing landscape that greatly affects drug development. Currently (2019), almost all of the key patents regarding phage display technologies have expired, including the Breitling/Dübel (EP0440147) and McCafferty/Winter (EP0774511, EP0589877) patents that expired in 2011 in Europe [149]. The US patents covering Dyax and Cambridge Antibody Technology phage antibody libraries have also reached the end of their 20-year protection period (Table 3). The expiration of these patents will allow more companies to create phage display human antibody libraries, advancing the march of therapeutic antibodies into the clinic. The lifting of intellectual property constraints will also spur academic institutions to translate developed phage-displayed antibodies into the clinic.
Many researchers have begun to take advantage of these free technologies. For example, Wayne Maraso and colleagues at the Dana-Farber Cancer Institute in Harvard University have constructed two phage display libraries containing 12 billion (Mehta I) and 15 billion (Mehta II) human naïve scFv antibody phages. These libraries have been used to identify numerous human scFv antibodies against a variety of targets [158, 159]. James Marks’ group has also established a phage-displayed human scFv library containing 6.7 billion members at the University of California, San Francisco [98]. This library has yielded a panel of specific antibodies for membrane proteins and living tissues with sub-nanomolar affinity [98, 160, 161]. We have also established a phage-displayed human naive scFv library at the Institute of Cellular and Organismic Biology (ICOB) in Academia Sinica in Taiwan. The antibody gene repertoires of the ICOB phage antibody library were isolated from the PBMCs of 50 healthy human donors, producing a library size of 60 billion individual scFv clones. This collection has been successfully used to select antibodies that bind a wide spectrum of target antigens, including pure recombinant proteins, glycans, cancer cells and virus particles [99, 103, 104].
Human antibody-producing mice
Transgenic animals provide a reliable platform for antibody drug development. Compared with other technologies for human antibody production, transgenic animals have several advantages, i.e., no need for humanization, more diversity, in vivo affinity maturation and clonal selection for antibody optimization. However, the large size of human Ig loci was a challenge during the development of transgenic mouse antibody technology. Additionally, the production of repertoires in transgenic mice that are similar or comparable those in humans requires diverse rearrangements combined with high expression of human V, D, and J segments [162]. To overcome these major challenges, different strategies have been successfully used to generate animals expressing human antibody repertoires (Table 4) [35, 36, 165].
Fully human antibody mice
The idea of producing human antibodies in transgenic mice was first suggested in 1985 when Alt et al. [166] proposed introducing human antibody genes into the mouse germline. This idea was unprecedented and provided a new direction for the development of human antibody production. In 1989, Brüggemann et al. [167] cloned the first human heavy chain construct, containing two human VH genes, for diversity segments (D) linked to the human heavy chain joining cluster (JH), and the μ constant region. The 25 kb construct was micro-injected as a mililocus plasmid into fertilized murine eggs, allowing its random insertion into the murine genome. About 4% of B lymphocytes expressed human μ chain at detectable levels in these transgenic mice. In addition, hybridomas of human IgM antibody could also be established using this transgenic strain. In 1992, Taylor et al. cloned the human κ light chain [168] construct, containing one human kappa light chain variable (Vκ) gene, the human kappa light chain joining cluster (Jκ) and kappa constant region (Cκ). While the mice expressed the human heavy chain (VH-D-JH-Cμ-Cγ1) and the human kappa light chain, the amount of human antibody was less than 10% of total antibodies, so the expression of human antibodies was not compatible with expression of mouse endogenous Ig [168].
At a similar time, various murine Ig knockout mouse strains were generated. In 1993, Chen et al. knocked out murine JH and Jκ genes with gene targeted deletion, inactivating mouse Ig [169, 170]. The human IgH and IgL transgenic mice were then crossed with murine IgH and IgL knockout mice in an attempt to create lines that could generate more diverse human antibodies. In 1994, the first human Ig -transgenic mice strain, HuMabMouse [35], was generated by Longberg et al. In this line, human IgH and IgΚ are expressed in murine IgH and IgΚ deficient mice. The entire human IgH genome is about 1.29 Mb and IgΚ is about 1.39 Mb, but the human Ig genome introduced into mice was less than 80 kb [35]. Since antibody diversity comes from germline V(D) J genes, it is reasonable that the introduction of more human variable genes will lead to more diversity of generated antibodies.
Likewise, Davies et al. [171] and Choi et al. [172] used yeast artificial chromosome (YAC) vectors in 1993 to respectively construct human IgΚ (~ 300 kb) and IgH (~ 85 kb) genes via yeast homologous recombination. In 1994, Green et al. [37] constructed human IgΚ (~ 170 kb) and IgH (~ 220 kb) genome YACs and successfully introduced them into mouse embryonic stem cells by yeast spheroplast-ES cell fusion. Furthermore, in 1997, Mendez et al. [36] introduced a larger human IgΚ (~ 700 Kb) or IgH (~ 1 Mb) YAC into mouse ES cells, crossing the human Ig mice with murine IgH and IgL knockout mice to generate XenoMouse [131]. As expected, the XenoMouse only express human antibodies rather than mouse antibodies [173]. While the development of this line has eliminated the interference from mouse endogenous Ig and expanded the human Ig genes, the efficiency of human antibody generation, Ig class-switching and somatic hypermutation still remain low, due to a lack of mouse constant region gene expression [158].
Chimeric human antibody mice
In order to overcome the drawbacks of a fully human heavy chain antibody, it is necessary to retain the original murine constant region. If a chimeric antibody with human Fab and murine Fc can be generated in mouse, the murine Fc would modulate the signaling for somatic hypermutation during antibody affinity maturation [158, 174, 175] and effector function of antibodies [174, 176]. Along these lines, Osborn et al. [163] linked human VH, D, and JH genes to the rat constant region locus in 2013. The large segments of human IgH and IgL were subcloned and linked by bacterial artificial chromosome (BAC) and YAC techniques, followed by micro-injection of the minilocus plasmid into fertilized rat oocytes. Meanwhile, endogenous rat Ig loci were silenced with a zinc finger nuclease. The resulting rat strain (OmniRat) chimeric human exhibits antibody production, antigen affinity and somatic mutations similar to wild-type rats. In 2014, Lee et al. [164] utilized BACs with Cre/loxP recombination in mouse ES cells to generate a mouse strain with human VH-D-JH and Vκ-Jκ inserted directly upstream of murine Cμ and Cκ regions (KyMouse). After antigen immunization, the KyMouse can process somatic hypermutation and produce high-affinity chimeric human antibodies. In another effort, Murphy et al. [165] used BACs to assemble large human Ig genes and serially micro-injected the constructs into mouse ES cells. The human IgH and IgL genes targeted and replaced the murine IgH and IgL, upstream of the constant region (VelocImmune mouse).
Development of successful antibody drugs from human antibody mice
Generation of human antibodies by transgenic animals has been accomplished by seven companies: Abgenix, XenoMouse (purchased by Amgen in 2005); Medarex, HuMAbMouse (purchased by Bristol Myers Squibb in 2009); Ligand, OmniRat; Kymab, KyMouse; Regeneron VelocImmune mouse; and the more recent Harbour Antibodies, H2L2 Mouse and Trianni Inc., Trianni Mouse [162, 165] (Table 4). The first antibody drug derived from transgenic mice was approved by the US FDA in 2006 [177], and as of 2019, 19 transgenic animal-derived antibody drugs [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34, 177] generated by Xenomouse, HuMabMouse, and VelocImmune mouse are on the market (Table 5). Eight of the drugs are used for cancer treatment, while the others are for autoimmune or inflammatory diseases.
There have been seven US FDA-approved antibody drugs generated from the XenoMouse (Table 5). In 2006, the first one, panitumumab (Vectibix, Amgen, human IgG2/kappa), was approved to treat the EGFR-expressing metastatic colorectal cancer with wild-type KRAS [188]. This mAb blocks the interaction of EGFR and its ligands, resulting in the inhibition of EGFR signaling and induction of cancer cell apoptosis. Two antibody drugs from the XenoMouse are used for autoimmune dermatologic diseases. One, secukinumab (Cosentyx, Novartis, human IgG1), binds to proinflammatory cytokine IL-17α to reduce inflammation in psoriasis [189]. The other is brodalumab (Siliq, Valeant Pharmaceuticals, human IgG2), which binds to the IL-17 receptor to inhibit the action of IL-17 family cytokines. The two mAb drugs were approved by the US FDA for psoriasis treatment in 2015 and 2017, respectively.
From the HuMabMouse, there have also been eight antibody drugs approved by the US FDA (Table 5). Two drugs, ipilimumab (Yervoy, Bristol-Myers Squibb, human IgG1) and nivolumab (Opdivo, Bristol-Myers Squibb, human IgG4/kappa), are used for melanoma treatment; the drugs were approved in 2011 and 2014, respectively. Ipilimumab binds to CTLA-4, an immune checkpoint inhibitor, blocking its interaction with B7 on APCs and causing cytotoxic T lymphocytes to kill cancer cells [190]. Nivolumab recognizes to PD-1, reducing inhibitory signaling to rehabilitate the immune response of tumor-specific T cells in patients [191]. Notably, nivolumab was also approved for non-small cell lung cancer treatment in 2018. Among the mAb drugs derived from the HuMabMouse, some are used for autoimmune diseases. For example, ustekinumab (Stelara, Johnson & Johnson, human IgG1/kappa) binds to cytokines, especially the p40 subunits of IL-12 and IL-23, blocking proinflammatory signaling to ease inflammation. This drug was approved for severe plaque psoriasis [17] in 2009 and for Crohn’s disease [192] in 2016.
The VelocImmune mouse is a second generation transgenic chimeric mouse and has yielded four approved drugs (Table 5). Dupilumab (Dupixent, Sanofi and Regeneron, human IgG4) binds to IL-4 receptor and inhibits the IL-4 and IL-13 pathway, as an eczema treatment. Sarilumab (Kevzara, Sanofi and Regeneron, human IgG1) inhibits IL-6 signaling by binding to the IL-6 receptor (IL-6R), which otherwise would upregulate the release of rheumatoid arthritis-related factors from hepatocytes. The two drugs were both approved in 2017. Notably, despite having access to the XenoMouse and owning Cambridge Antibody Technology (the phage display company behind Humira), AstraZeneca paid over $120 million for a few breeding pairs of VelocImmune mice [193].
To improve the diversity of products and generate better antibody drugs, major development efforts have yielded models, such as the fully human antibody mouse and second-generation chimeric human antibody mice, over the 30 years since the first transgenic mouse was generated in 1989 [167]. The continued refinement and advancement of transgenic animals provides ever more possibilities for antibody drug development by global pharmaceutical factories.
Single B cell antibody technology
In the human immune system, antibody responses are robust, highly specific, neutralizing and self-tolerant. Producing therapeutic human antibodies using the traditional hybridoma technique or transgenic mice requires long-term immunization procedures and screening, while the clinical use of murine antibodies may trigger severe immunogenic responses (such as HAMA) [194]. To avoid these obstacles, a technique for immortalizing human B cells with Epstein-Barr virus was developed [195,196,197]. This method is useful in certain situations, but it suffers from drawbacks, such as inefficiency in some patients and difficulties maintaining the stability of some transformed clones. While mice carrying human Ig genes have been created [35, 36, 163,164,165], the immune reactivity of these mice often cannot be triggered as robustly as natural human antibody responses. Thus, in emergent cases such as infectious diseases, single B cell antibody technologies have the major advantage of requiring only a few cells, allowing the highly efficient and rapid isolation of potential mAbs. Moreover, single B cell cloning preserves the biologically mediated heavy chain and light chain pairing, instead of the random pairing that is characteristic of mAbs from phage display antibody libraries. These randomly paired mAbs occasionally lose binding affinity or develop self-reactivity when transferred from scFv to intact IgG formats.
Identification and isolation of single B cells
Single B cells can be isolated from either PBMCs or lymphoid tissues using micromanipulation [198, 199], laser capture microdissection [200], and fluorescence-activated cell sorting [199, 201, 202]. Generally, mononuclear cells are purified from PBMCs or bone marrow by Ficoll-Paque density gradient centrifugation. Based on B cell expression of specific cell surface markers in different stages, isolation of single B cells by fluorescence-activated cell sorting is widely utilized, especially in the identification of rare and discrete B cell subpopulations. Antigen-coated magnetic beads [203] and fluorescence-conjugated antigens [204,205,206] are also often used to select antigen-specific B cells in a process known as antigen baiting. Neutralizing human mAbs against Puumala virus were generated from B cells isolated using antigen-coated magnetic beads [207]. Recently, antigen-conjugated fluorescent beads have been used to identify antigen-specific B cells [208]. Fluorescent virus-like particles of rotavirus served as antigen bait for single RV-specific B cells, which had been extracted from healthy rotavirus-infected infants or adult donors [209]. HIV envelope protein antigens have also been used to isolate antibodies that broadly neutralize HIV-1 [210, 211]. Moreover, isolation of dengue virus-specific memory B cells was reported [212, 213]. Thus, antigen baiting may be applied as a preliminary selection tool to be used on a polyclonal mixture.
Cloning of single B cells and screening of antibodies
After single B cell sorting, direct cloning of each Ig heavy chain and corresponding light chain should be performed [201, 202]. This step involves the use of nested or semi-nested reverse transcription-polymerase chain reaction (RT-PCR) for amplification of the variable heavy and light chain of each identified B cell. Usually, forward primers are directed toward IgH and IgL variable leader sequences and reverse primers are complementary to the Ig constant region [201, 214]. By optimizing different primer-set mixtures, the recoveries of the VH and VL may be improved [201, 215]. The genes are then cloned and expressed in mammalian cell lines for the immediate generation of recombinant mAbs. Following the detection of mAb reactivity, the characteristics of each generated mAb are determined. Furthermore, for high-throughput screening and evaluation of secreted mAbs with ideal reactivity, a cell-based microarray chip system [216] and microengraving techniques [217,218,219,220] have been described. The cell-based microarray chip system, immunospot array assay on a chip, enables the trapping of secreted antibodies by a chip that is coated with antibody against Ig, therefore, it is used to identify and recover specific antibody-secreting cells [216]. The microengraving method depends on the use of a soft lithographic technique to generate microarrays comprising the secreted antibodies of single cells [217]. These two approaches offer the advantages of early and rapid identification of clones with high affinity and specificity to the antigen of interest.
Generation of human antibodies by single B cell
In the face of threats from novel emergent pathogens, the rapid development of immunotherapies or insights into the diversity of antibody repertoire are beneficial, and single B cell sorting provides a highly efficient technology to achieve these goals. In the past, human mAbs have been generated by the single B cell method for bacterial, parasitic, virus infected or autoimmune diseases.
Among bacteria, Bacillus anthracis is one of the most concerning species. B. anthracis is a fatal pathogen that causes severe anthrax disease in humans and has been used as a biological weapon. Although antibiotics are available for anthrax treatment and as post-exposure prophylaxis, anti-anthrax protective antibodies from single human B cells will still be a crucial addition to the treatment toolkit [221, 222]. In an example targeting yeast infections, anti-Candida mAbs antibodies derived by the single human B cell method can enhance phagocytosis to protect against disseminated candidiasis [223].
The single B cell method has also successfully yielded anti-viral mAbs. Rapid isolation of dengue-neutralizing antibodies from human antigen-specific memory B-cell cultures [224] and characterization of antigen-specific B cells in the peripheral blood of DENV-immune individuals [213] were both reported. In another example, Iizuka and colleagues described the identification of cytomegalovirus pp65 antigen-specific human mAbs using single B cell-based antibody gene cloning [225]. For rotavirus, the single B cell method was performed to analyze the rotavirus antibody gene repertoire of VP6-specific B cells in naive and memory B cell subsets [226] and generate rotavirus-specific human mAbs by sorting single B cells from small-intestinal mucosa [227]. Human mAbs against zika virus NS1 have also been generated by the single B cell method [228]. Besides mAbs for bacterial and virus infection, the single human B cell method has also yielded a complement factor H (CFH) therapeutic antibody for cancer. The recombinant anti-CFH antibody can induce complement-mediated cytotoxicity (CDC) through complement activation and release of anaphylatoxins [229].
Development of single B cell-derived antibodies in clinical trials
Many virus-targeting mAbs are also currently in clinical trials. For example, Ebolavirus is a highly lethal pathogen that causes 25–90% mortality in humans. Therapeutic mAbs for ebolavirus infection have been derived from B cells of vaccinated human donors or survivors [230,231,232]. Impressively, human mAb114, which is derived from sorted memory B cells targeted to the Zaire ebolavirus glycoprotein, protects macaques when administered as late as 5 days after challenge [231]. Clinical trials for this drug are at Phase I (NCT03478891), Phase II and III (NCT03719586).
Acquired immunodeficiency syndrome is caused by HIV, and an estimated 36.9 million people worldwide are infected. HIV-1 envelope protein is an attractive therapeutic target for antibody and vaccine design. Five human mAbs against anti-HIV envelope protein have been generated by the single B cell approach and are under evaluation in clinical trials (3BNC117, Phase I/II; 10–1074, Phase I; VRC01, Phase I/II; PGT121, Phase I/II and N6, Phase I) [233]. A Phase I clinical trial (NCT02579083) is also being conducted on the prevention of sexual transmission of HIV-1 and herpes simplex virus by MB 66 combined with an anti-herpes simplex virus antibody (AC8) and an anti-HIV antibody (VRC01).
Different influenza viruses cause epidemics ever year, and influenza vaccines are the most useful measure to prevent seasonal influenza. Single B cell isolation for the generation of potent and broadly neutralizing anti-influenza antibodies has become a popular undertaking [234, 235]. MHAA4549A, a human mAb targeting the hemagglutinin stalk of influenza A virus was cloned from a single human plasmablast cell from an influenza virus vaccinated donor [236]. A Phase II clinical trial of MHAA4549A as a monotherapy for acute uncomplicated seasonal influenza A in otherwise healthy adults was recently completed (NCT02623322). CT P27, which contains two human mAbs (CT P22 and CT P23), was created by Celltrion and is in Phase II (NCT03511066). RG 6024, also named MHAB5553A, was generated by Genentech with a modified version of the single B cell isolation method [237]; it is currently under examination in Phase I (NCT02528903) trial. The Phase II trial for another antibody, TCN 032, was stopped in 2012 (NCT01719874).
Profiling of respiratory syncytial virus (RSV) antibody repertoires from the memory B cells of naturally infected adults [238] or generation of neutralizing antibodies from RSV-infected infants [239] has been undertaken as well. MEDI8897, an anti-RSV antibody developed by MedImmune, is currently being evaluated for safety and efficacy in Phase II clinical trial (NCT02878330).
Over the past decade, generation of mAbs by single B cell technology has become increasingly attractive. However, there are still no US FDA-approved therapeutic mAbs developed by this method that is used for clinical treatment of any disease. Although single B cell technology possesses several irreplaceable advantages, there are still challenges to be overcome. For example, the antigen labeling technique, the configuration of sorting antigens (e.g., monomer or dimer) and the design of primer sets are all important considerations for successful generation of mAbs. In the future, recovery of mAbs from single B cell platforms may be a powerful tool in combination with next generation sequencing for development of novel diagnostics, pharmacokinetic applications, and clinical therapeutics.
Affinity maturation of antibodies
Antibodies identified from humanized, phage or transgenic methods are often further engineered, including the replacement of residues with binding liabilities in the binding region. In addition, point mutations in the antibody structure sometimes result in products with weaker antigen interactions than the original antibody (low affinity), but some mutations will result in stronger interactions (high affinity). The process of enhancing affinity for antigens is called affinity maturation. After V(D) J recombination, affinity maturation occurs in mature B cells with the help from helper T cells.
Affinity maturation is an important characteristic of the humoral immune response, which can result in antibodies with low picomolar affinity [240, 241]. High affinity is a crucial attribute of antibodies for the neutralization of cytokines or growth factor-induced signaling. Generally, for a mAb to be considered for therapeutic drug development, it should have an affinity of 1 nM or less for the target antigen [242]. Moreover, humanization of mouse mAbs frequently reduces affinity [243]. Thus, the use of affinity maturation is often a necessary step in antibody drug development [244].
Approaches for affinity maturation
Phage display and yeast display have been widely used for affinity maturation of antibodies, due to their amenability to easily screen for high affinity variants and to high throughput applications [81]. Methods for increasing antibody affinity can be broadly divided into two broad categories. The first is to generate a large randomly mutated library of CDR or entire variable domain sequences, followed by a selection of higher affinity variants from this large number of mutants. Another approach is to prepare small libraries by focused mutagenesis or hotspot mutagenesis that mimics in vivo affinity maturation. In this focused method, a high affinity variant is selected to be randomized at individual positions in each of the six CDRs or at a discrete point in the variable domain called the hotspot. The usual practice is to combine different mutations, resulting in small increases in affinity. The combination of these different mutations may have an additive or synergistic effect that can result in substantial increases in the affinity of the antibody to the antigen [245]. Phage display technology can be employed to identify high affinity antibodies in an antibody gene mutation library under stringent biopanning conditions, including decreased antigen amount, extended incubation and intensive washing steps or by competition with soluble antigen [104, 246]. Using phage display with VH and VL CDR3 mutation libraries, the affinity of anti-HER2 antibody was improved more than 1200-fold [97].
Diversification of antibody genes is the initial step of affinity maturation in vitro, and this step may be achieved using various strategies, such as random mutations, targeted mutations or chain shuffling [246]. Mutations can be randomly introduced into the variable regions of antibody genes by error-prone PCR in mutator E. coli strains [247, 248]. Chain shuffling approaches are those in which one of two chains, VH and VL, is fixed and recombined with a repertoire of partner chains to produce a next-generation library [249]. Moreover, mutations can be introduced to particular regions of the antibody gene. This type of targeting mutation approach was employed to diversify CDR residues and was shown to be effective in ameliorating the affinity of antibodies [250]. Therefore, this method is more relevant to in vivo somatic mutations during B cell evolution because mutations accumulate more efficiently in the CDR than framework residues.
Future perspectives
The field of therapeutic antibodies has undergone rapid growth in recent years, becoming a dominant force in the therapeutics market. However, there is still significant growth potential for the therapeutic antibody field. Traditionally, antibodies have been used for the treatment of cancer, autoimmune diseases, and infectious diseases. If the molecular mechanisms of a specific disease can be clearly elucidated and the specific proteins or molecules involved in pathogenesis can be identified, antibodies may provide an effective therapeutic option. For example, anti-CGRP receptor antibodies (erenumab, galcanezumab, or fremanezumab) have been developed for the prevention of migraine. Anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies (evolocumab or alirocumab) are used for the treatment of hypercholesterolemia. Anti-fibroblast growth factor 23 (FGF23) antibody (burosumab) is used to treat X-linked hypophosphatemia. Anti-IL6R antibody (sarilumab and tocilizumab) can be used for the treatment of rheumatoid arthritis. Anti-Factor IXa/Xa antibody (emicizumab) is a valuable treatment for hemophilia A. Anti-von Willebrand factor antibody (caplacizumab) is approved for the treatment of thrombotic thrombocytopenic purpura, and other antibodies will be approved for new indications in the near future.
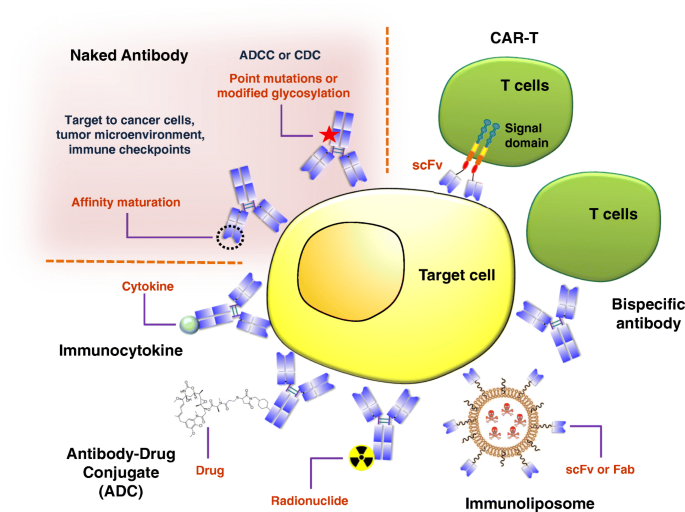
Therapeutic antibodies can roughly be separated into two broad categories (Fig. 5). In the first category, the naked antibody is directly used for disease therapy. Cancer treatments from this category may act through several different mechanisms, including mediated pathways (e.g., ADCC/CDC), direct targeting of cancer cells to induce apoptosis, targeting the tumor microenvironment, or targeting immune checkpoints. In mediated pathways, the antibody kills cancer cells by recruiting natural killer cells or other immune cells. Recently, new technological developments have been made to enhance the therapeutic effects of ADCC or CDC, such as antibody Fc point mutations [251,252,253] or modification of glycosylation [254,255,256,257,258] to improve cancer cell killing capabilities. The direct induction apoptosis in cancer cells has traditionally been the preferred mechanism for therapeutic antibodies. With regard to targeting the tumor microenvironment, antibodies can inhibit tumorigenesis by targeting factors involved in cancer cell growth. For example, Avastin targets vascular endothelial growth factor (VEGF) to inhibit blood vessel growth around the tumor, shutting down the supply of nutrients required for the cancer cell growth. Immune checkpoints have proven to be valuable targets for cancer treatment. In the future, studies evaluating synergistic effects of antibodies and chemotherapeutic drugs, radiotherapy or other biologic agents will greatly benefit the further development of antibody therapeutics. Furthermore, the identification of novel biomarkers may improve the efficacy and specificity of antibody-based therapy for human diseases.
Schematic overview showing the development of antibody-based therapeutics for the treatment of cancer. Therapeutic antibodies can be roughly separated into two broad categories. The first category involves the direct use of the naked antibody for disease therapy. Antibodies in this category are used for cancer treatment and elicit cell death by different mechanisms, including ADCC/CDC, direct targeting of cancer cells to induce apoptosis, targeting the tumor microenvironment, or targeting immune checkpoints. For antibodies in the second category, additional engineering is performed to enhance their therapeutic efficacy. Some general approaches for the use of these antibodies include immunocytokine, antibody-drug conjugate (ADC), antibody-radionuclide conjugate (ARC), bispecific antibody, immunoliposome, and CAR-T
In the second category of antibody drugs, additional modifications are made to the antibody in order to enhance its therapeutic value. Some general approaches include immunocytokines, antibody-drug conjugate, antibody-radionuclide conjugates, bispecific antibodies, immunoliposomes, and chimeric antigen receptor T cell (CAR-T) therapy. To create an immunocytokine, a selected cytokine is fused to an antibody to enhance delivery specificity [259]. Antibody drug conjugates consist of an antibody that targets a cancer-specific marker conjugated to the small molecule drug; the antibody enhances delivery to the tumor site, increasing the efficacy of the small molecule while reducing side effects by reducing non-specific toxicity to non-target tissues [260]. The antibody may also be conjugated to a radionuclide, in order to direct radiotherapy more specifically to the tumor site [261]. For bispecific antibodies, antibodies targeting two receptors are engineered to further enhance therapeutic effects [262]. Antibody-engaged effector cell functions may enhance the therapeutic efficacy of bispecific antibodies. With regard to immunoliposomes, the binding site of the antibody (scFv or Fab) is cleaved from the constant region and subsequently conjugated to different nano-drug delivery systems, such as liposomal drugs, to provide more specific targeting [263, 264]. Lastly, CAR-T involves inserting the gene for a chimeric T cell receptor-antibody targeting a specific cancer marker into T cells, such that the engineered cells target and kill cancer cells [265, 266]. In recent years, this approach has garnered major attention from the scientific and medical community due to its significant clinical benefits to cancer patients. In many cases, patients have experienced complete remission or even been completely cured of cancer [267,268,269,270,271].
Although new methods have been well-established for generating fully human antibodies, such as human antibody transgenic mice and human single B cell antibody techniques, phage display still has advantages as an antibody drug discovery platform, based on its efficient and economical in vitro selection methodology. Recently, some advanced techniques have been applied in antibody discovery, including high-throughput robotic screening [272], next generation sequencing [273] and single cell sequencing [274, 275]. These techniques are expected to greatly accelerate the identification of specific phage binders, facilitating mAb development for use in research, clinical diagnostics, and pharmaceuticals for the treatment of human disease.
By reviewing currently approved mAbs, one may easily see how sophisticated formats were developed in response to challenges posed by therapeutic indications. These mAb engineering solutions are highlighted by antibody-drug conjugates, glycoengineered mAbs, immunomodulators, bispecific mAbs, and CAR-T cells.
Conclusions
Here, we summarize five technical platforms that are related to the production of therapeutic antibodies, including chimeric antibodies, humanization, phage display, transgenic mice, and single B cell antibody technology (Fig. 3d). Phage display, transgenic mice, and single B cell antibody technology have proven to be reliable methods for the generation of human antibodies. As enormous storehouses of antibody encoding genes (> 1010) with unknown properties, high quality (antibody diversity) phage antibody libraries are critical to the successful identification of therapeutic mAbs. In addition, an optimal selection from phage display libraries is dependent on target antigen quality, antigen immobilization, and tight control of binding and wash conditions. Furthermore, careful pre-screening design of conditions can tailor the characteristics of antibodies discovered from biopanning, including conformation specificity, epitope specificity, internalization, neutralization, and interspecies cross-reactivity. Currently, there are nine fully human antibodies that were discovered from phage libraries approved for therapy, and dozens more phage-derived antibody therapeutics are in clinical trials, waiting to enter the market [149] (Table 5).
In order to improve the quality of antibody drugs, researchers have developed several transgenic animals, including fully human mice and second-generation human chimeric mouse. The continued refinement and advancement of transgenic animals provide more options for antibody drug development in global pharmaceutical factories. All the transgenic-derived mAbs approved for therapeutic use have come from from three companies: Abgenix (XenoMouse) [36], Medarex (HuMAbMouse) [35], and Regeneron (VelocImmune) [165]. Depending on the immunization protocol, high affinity human antibodies can be obtained through selection of the clones generated in the animals. This selection is mainly accomplished by hybridoma technology. Currently, there are 19 approved human mAbs that were discovered from these three transgenic animals (Table 5).
Over the past decade, generation of mAbs from isolated single B cells has become an increasingly attractive approach. To date, no US FDA-approved therapeutic mAbs have been derived from this method; however, it possesses several major advantages, and ongoing challenges are currently being solved. The success of the method relies heavily on the antigen labeling technique, the configuration of sorting antigens (e.g., monomer or dimer) and the set of primers used for amplification. In the future, the recovery of mAbs from single B cells is expected to become a powerful tool in combination with next generation sequencing for diagnostics, pharmacokinetic application, and clinical therapeutics.
As a result of highly active development of antibody drugs in recent decades, mAbs have emerged among the major class of therapeutic agents for the treatment of many human diseases, especially cancers, immunological, infectious, neural and metabolic diseases. Sales growth and regulatory approval of mAb products were slow until the late 1990s when the first chimeric mAbs were approved (annual sales of $0.3 billion in 1997). With the subsequent approval of humanized and then fully human mAbs, the rate of product approvals and sales of mAb products has increased rapidly, with global sales revenue for all mAb products at $115.2 billion in 2018 (Fig. 1) [276]. The continued growth of mAb products in the coming years is expected to be a major driver of overall biopharmaceutical product sales.
Availability of data and materials
Not applicable.
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- BAC:
-
Bacterial artificial chromosome
- BLYS:
-
B lymphocyte stimulator
- CALCRL:
-
Calcitonin receptor-like receptor
- CAR-T:
-
Chimeric antigen receptor T cell
- CDC:
-
Complement-mediated cytotoxicity
- CDR:
-
Complementary-determining region
- CGRP:
-
Calcitonin gene-related peptide
- CLL:
-
Chronic lymphocytic leukemia
- CoV:
-
Coronavirus
- CSC:
-
Cancer stem cell
- CTLA-4:
-
Cytotoxic T-lymphocyte–associated antigen 4
- Cκ:
-
Κappa constant region
- D:
-
Diversity segment
- EGFR:
-
Epidermal growth factor receptor
- ES cell:
-
Embryonic stem cell
- Fab:
-
Antigen-binding fragment
- Fc:
-
Fragment crystallizable region
- HAMA:
-
Human anti-mouse antibody
- HER2:
-
Human epidermal growth gactor receptor 2
- HIV:
-
Human immunodeficiency virus
- ICOB:
-
Institute of Cellular and Organismic Biology
- Ig:
-
Immunoglobulin
- JH :
-
Heavy chain joining cluster
- Jκ:
-
Kappa chain joining cluster
- mAb:
-
Monoclonal antibody
- MERS:
-
Middle East respiratory syndrome
- MM:
-
Multiple myeloma
- MSI-H:
-
Microsatellite instability high
- NSCLC:
-
Non-small cell lung cancer
- PBMC:
-
Peripheral blood mononuclear cell
- PCR:
-
Polymerase chain reaction
- PD-1:
-
Programmed cell death protein 1
- PD-L:
-
Programmed death-ligand
- RAMP1:
-
Receptor activity-modifying protein 1
- RSV:
-
Respiratory syncytial virus
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- scFv:
-
Single chain fragment variable
- SLE:
-
Systemic lupus erythematosus
- TNFα:
-
Tumor necrosis factor α
- US FDA:
-
United States Food and Drug Administration
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- VH :
-
Variable heavy chain
- VL :
-
Variable light chain
- Vκ:
-
Kappa variable light chain
- YAC:
-
Yeast artificial chromosome
References
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7.
Kaplon H, Reichert JM. Antibodies to watch in 2019. MAbs. 2019;11:219–38.
The Antibody Society. In: Approved antibodies. Jun 27, 2019 https://www.antibodysociety.org/ Accessed 15 Jul 2019.
Lefranc MP. IMGT, the International ImMunoGeneTics Information System. Cold Spring Harb Protoc. 2011;2011:595–603.
Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14.
Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984;81:6851–5.
Foster RH, Wiseman LR. Abciximab. An updated review of its use in ischaemic heart disease. Drugs. 1998;56:629–65.
Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–95.
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:3266–74.
Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–5.
Tsurushita N, Hinton PR, Kumar S. Design of humanized antibodies: from anti-Tac to Zenapax. Methods. 2005;36:69–83.
Watier H, Reichert J. Evolution of antibody therapeutics. In: Vaughan T, Osbourn J, Jallal B, editors. Protein terapeutics. Weinheim: Wiley-VCH Verlag GmbH & Co, KGaA; 2017. p. 25–49.
McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–4.
Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7.
Wu CH, Liu IJ, Lu RM, Wu HC. Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci. 2016;23:8.
Kempeni J. Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7. Ann Rheum Dis. 1999;58(Suppl 1):I70–2.
Bartlett BL, Tyring SK. Ustekinumab for chronic plaque psoriasis. Lancet. 2008;371:1639–40.
Church LD, McDermott MF. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr Opin Mol Ther. 2009;11:81–9.
Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47:383–96.
Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111:1094–100.
Reddy GK, Nadler E, Jain VK. Denosumab (AMG 162), a Fully Human Monoclonal Antibody Against RANK Ligand Activity. Support Cancer Ther. 2005;3:14–5.
Morse MA. Technology evaluation: ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther. 2005;7:588–97.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33.
Roth EM, Diller P. Alirocumab for hyperlipidemia: physiology of PCSK9 inhibition, pharmacodynamics and Phase I and II clinical trial results of a PCSK9 monoclonal antibody. Futur Cardiol. 2014;10:183–99.
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8.
Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk--primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–82.
Chioato A, Noseda E, Stevens M, Gaitatzis N, Kleinschmidt A, Picaud H. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vacc Immunol: CVI. 2012;19:1597–602.
Chiorean EG, Sweeney C, Youssoufian H, Qin A, Dontabhaktuni A, Loizos N, et al. A phase I study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRalpha) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:595–604.
Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66.
Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308.
Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, et al. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73:1626–34.
Tepper S, Ashina M, Reuter U, Brandes JL, Dolezil D, Silberstein S, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–34.
Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379:341–51.
Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368:856–9.
Mendez MJ, Green LL, Corvalan JR, Jia XC, Maynard-Currie CE, Yang XD, et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Genet. 1997;15:146–56.
Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994;7:13–21.
Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–86.
Gibson TB, Ranganathan A, Grothey A. Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6:29–31.
Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18:585–608.
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–21.
Gokbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N Engl J Med. 2017;377:809–18.
Kyowa Hakko Kirin Co. Ltd. Consolidated Financial Summary (IFRS) Fiscal 2018. 2019, February 5.
Grilo AL, Mantalaris A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol. 2019;37:9–16.
Donini C, D'Ambrosio L, Grignani G, Aglietta M, Sangiolo D. Next generation immune-checkpoints for cancer therapy. J Thorac Dis. 2018;10:S1581–S601.
Sator P. Safety and tolerability of adalimumab for the treatment of psoriasis: a review summarizing 15 years of real-life experience. Ther Adv Chronic Dis. 2018;9:147–58.
Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013;72:517–24.
Zamora-Atenza C, Diaz-Torne C, Geli C, Diaz-Lopez C, Ortiz MA, Moya P, et al. Adalimumab regulates intracellular TNFalpha production in patients with rheumatoid arthritis. Arthritis Res Ther. 2014;16:R153.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–9.
Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21:4286–93.
Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–52.
Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83.
Velcheti V, Chandwani S, Chen X, Pietanza MC, Burke T. First-line pembrolizumab monotherapy for metastatic PD-L1-positive NSCLC: real-world analysis of time on treatment. Immunotherapy. 2019;11:889–901.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–33.
Morgensztern D, Herbst RS. Nivolumab and Pembrolizumab for Non-Small Cell Lung Cancer. Clin Cancer Res. 2016;22:3713–7.
US Food and Drug Administration. FDA approves novel preventive treatment for migraine. 2018, May 17.
Paemeleire K, MaassenVanDenBrink A. Calcitonin-gene-related peptide pathway mAbs and migraine prevention. Curr Opin Neurol. 2018;31:274–80.
Liang YL, Khoshouei M, Deganutti G, Glukhova A, Koole C, Peat TS, et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature. 2018;561:492–7.
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–50.
Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–44.
Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555–61.
Emu B, Fessel J, Schrader S, Kumar P, Richmond G, Win S, et al. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N Engl J Med. 2018;379:645–54.
Sanofi EL. FDA to review isatuximab as a potential treatment for relapsed/refractory multiple myeloma. Paris, 2019.
Richardson PG, Attal M, Campana F, Le-Guennec S, Hui AM, Risse ML, et al. Isatuximab plus pomalidomide/dexamethasone versus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma: ICARIA Phase III study design. Future Oncol. 2018;14:1035–47.
Novartis AG. Novartis Financial Results – Q2 2018. July 18, 2018 https://www.novartis.com/news/novartis-financial-results-q2-2018 Accessed 15 Jul 2019.
TESARO Inc. TESARO Announces Data Presentations at ESMO 2018 Congress. Oct 20, 2018 https://www.globenewswire.com/news-release/2018/10/20/1624331/0/en/TESARO-Announces-Data-Presentations-at-ESMO-2018-Congress.html Accessed 15 Jul 2019.
TG Therapeutics I. TG Therapeutics announces update regarding UNITY-CLL Phase 3 trial. Sep 25, 3018 http://ir.tgtherapeutics.com/news-releases/news-release-details/tg-therapeutics-announces-update-regarding-unity-cll-phase-3 Accessed 15 Jul 2019.
Gorman SD, Clark MR. Humanisation of monoclonal antibodies for therapy. Semin Immunol. 1990;2:457–66.
Mountain A, Adair JR. Engineering antibodies for therapy. Biotechnol Genet Eng Rev. 1992;10:1–142.
Queen C, Schneider WP, Selick HE, Payne PW, Landolfi NF, Duncan JF, et al. A humanized antibody that binds to the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1989;86:10029–33.
Choi Y, Hua C, Sentman CL, Ackerman ME, Bailey-Kellogg C. Antibody humanization by structure-based computational protein design. MAbs. 2015;7:1045–57.
Olimpieri PP, Marcatili P, Tramontano A. Tabhu: tools for antibody humanization. Bioinformatics. 2015;31:434–5.
Swindells MB, Porter CT, Couch M, Hurst J, Abhinandan K, Nielsen JH, et al. abYsis: integrated antibody sequence and structure—management, analysis, and prediction. J Mol Biol. 2017;429:356–64.
Abhinandan KR, Martin ACR. Analyzing the “Degree of Humanness” of Antibody Sequences. J Mol Biol. 2007;369:852–62.
Pelat T, Bedouelle H, Rees AR, Crennell SJ, Lefranc M-P, Thullier P. Germline Humanization of a Non-human Primate Antibody that Neutralizes the Anthrax Toxin, by in Vitro and in Silico Engineering. J Mol Biol. 2008;384:1400–7.
Thullier P, Huish O, Pelat T, Martin ACR. The Humanness of Macaque Antibody Sequences. J Mol Biol. 2010;396:1439–50.
Gao SH, Huang K, Tu H, Adler AS. Monoclonal antibody humanness score and its applications. BMC Biotechnol. 2013;13:55.
Ducancel F, Muller BH. Molecular engineering of antibodies for therapeutic and diagnostic purposes. MAbs. 2012;4:445–57.
Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2:256–65.
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325.
Waldmann H. Human Monoclonal Antibodies: The Benefits of Humanization. Methods Mol Biol. 1904;2019:1–10.
Rebello PRUB, Hale G, Friend PJ, Cobbold SP, Waldmann H. Anti-globulin responses to rat and humanized campath-1 monoclonal antibody used to treat transplant rejection1. Transplantation. 1999;68:1417–9.
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48.
Jackisch C, Kim SB, Semiglazov V, Melichar B, Pivot X, Hillenbach C, et al. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26:320–5.
Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. mAbs. 2010;2:256–65.
Bender NK, Heilig CE, Dröll B, Wohlgemuth J, Armbruster F-P, Heilig B. Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatol Int. 2007;27:269–74.
West RL, Zelinkova Z, Wolbink GJ, Kuipers EJ, Stokkers PC, van der Woude CJ. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn's disease. Aliment Pharmacol Ther. 2008;28:1122–6.
Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:964–75.
Parmley SF, Smith GP. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988;73:305–18.
Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–8.
Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996;14:309–14.
Breitling F, Dubel S, Seehaus T, Klewinghaus I, Little M. A surface expression vector for antibody screening. Gene. 1991;104:147–53.
Barbas CF, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991;88:7978–82.
Nixon AE, Sexton DJ, Ladner RC. Drugs derived from phage display: from candidate identification to clinical practice. MAbs. 2014;6:73–85.
Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindqvist E, Schier R, et al. Efficient construction of a large nonimmune phage antibody library: The production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci U S A. 1998;95:6157–62.
Lu RM, Chang YL, Chen MS, Wu HC. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials. 2011;32:3265–74.
Bradbury ARM, Sidhu S, Dübel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol. 2011;29:245–54.
Lerner RA. Combinatorial antibody libraries: new advances, new immunological insights. Nat Rev Immunol. 2016;16:498–508.
Weber M, Bujak E, Putelli A, Villa A, Matasci M, Gualandi L, et al. A highly functional synthetic phage display library containing over 40 billion human antibody clones. PLoS One. 2014;9:e100000.
Lee TY, Wu HC, Tsao TC and Lin W; Fountain Biopharma Inc., assignee. Antibodies to interleukin-6 US patent US 9,234,035. 2016 January 12.
Wu HC, Lu RM, Chiu CY, Liu IJ and Chang YL; Academia Sinica, assignee. Anti-vascular endothelial growth factor receptor 2 (VEGFR2) antibody and methods of use thereof for detecting VEGFR2 and for inhibiting tumor growth, tumor angiogenesis and/or inducing cancer cell cytotoxicity. US patent US10,196,447. 2018 February 5.
de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–30.
Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86.
Rothe C, Urlinger S, Lohning C, Prassler J, Stark Y, Jager U, et al. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol. 2008;376:1182–200.
Chan CE, Lim AP, MacAry PA, Hanson BJ. The role of phage display in therapeutic antibody discovery. Int Immunol. 2014;26:649–57.
Chan CE, Chan AH, Lim AP, Hanson BJ. Comparison of the efficiency of antibody selection from semi-synthetic scFv and non-immune Fab phage display libraries against protein targets for rapid development of diagnostic immunoassays. J Immunol Methods. 2011;373:79–88.
Wrublewski DT. Analysis for Science Librarians of the 2018 Nobel Prize in Chemistry: Directed Evolution of Enzymes and Phage Display of Peptides and Antibodies. Sci Technol Libr. 2019:1–19.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124.
Swaminathan SK, Niu L, Waldron N, Kalscheuer S, Zellmer DM, Olin MR, et al. Identification and characterization of a novel scFv recognizing human and mouse CD133. Drug Deliv Transl Res. 2013;3:143–51.
Nilvebrant J, Kuku G, Bjorkelund H, Nestor M. Selection and in vitro characterization of human CD44v6-binding antibody fragments. Biotechnol Appl Biochem. 2012;59:367–80.
Baskar S, Suschak JM, Samija I, Srinivasan R, Childs RW, Pavletic SZ, et al. A human monoclonal antibody drug and target discovery platform for B-cell chronic lymphocytic leukemia based on allogeneic hematopoietic stem cell transplantation and phage display. Blood. 2009;114:4494–502.
Zhu X, Bidlingmaier S, Hashizume R, James CD, Berger MS, Liu B. Identification of internalizing human single-chain antibodies targeting brain tumor sphere cells. Mol Cancer Ther. 2010;9:2131–41.
Larsen SA, Meldgaard T, Fridriksdottir AJ, Lykkemark S, Poulsen PC, Overgaard LF, et al. Selection of a breast cancer subpopulation-specific antibody using phage display on tissue sections. Immunol Res. 2015;62:263–72.
Sun Y, Shukla GS, Weaver D, Pero SC, Krag DN. Phage-display selection on tumor histological specimens with laser capture microdissection. J Immunol Methods. 2009;347:46–53.
Larsen SA, Meldgaard T, Lykkemark S, Mandrup OA, Kristensen P. Selection of cell-type specific antibodies on tissue-sections using phage display. J Cell Mol Med. 2015.
Su Y, Bidlingmaier S, Lee N-K, Liu B. Combine phage antibody display library selection on patient tissue specimens with laser capture microdissection to identify novel human antibodies targeting clinically relevant tumor antigens. In: Hust M, Lim TS, editors. Phage Display: Methods and Protocols. New York: Springer New York; 2018. p. 331–47.
Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–70.
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410.
Caracciolo G. Clinically approved liposomal nanomedicines: lessons learned from the biomolecular corona. Nanoscale. 2018;10:4167–72.
Becerril B, Poul MA, Marks JD. Toward selection of internalizing antibodies from phage libraries. Biochem Biophys Res Commun. 1999;255:386–93.
Roth A, Drummond DC, Conrad F, Hayes ME, Kirpotin DB, Benz CC, et al. Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol Cancer Ther. 2007;6:2737–46.
Maruyama T, Parren PW, Sanchez A, Rensink I, Rodriguez LL, Khan AS, et al. Recombinant human monoclonal antibodies to Ebola virus. J Infect Dis. 1999;179(Suppl 1):S235–9.
Flego M, Di Bonito P, Ascione A, Zamboni S, Carattoli A, Grasso F, et al. Generation of human antibody fragments recognizing distinct epitopes of the nucleocapsid (N) SARS-CoV protein using a phage display approach. BMC Infect Dis. 2005;5:73.
Kang X, Yang BA, Hu Y, Zhao H, Xiong W, Yang Y, et al. Human neutralizing Fab molecules against severe acute respiratory syndrome coronavirus generated by phage display. Clin Vacc Immunol: CVI. 2006;13:953–7.
Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–34.
Lerner RA. Manufacturing Immunity to Disease in a Test Tube: The Magic Bullet Realized. Angew Chem Int Ed. 2006;45:8106–25.
Zhang X, Qi X, Zhang Q, Zeng X, Shi Z, Jin Q, et al. Human 4F5 single-chain Fv antibody recognizing a conserved HA1 epitope has broad neutralizing potency against H5N1 influenza A viruses of different clades. Antivir Res 2013;99:91–9.
Kashyap AK, Steel J, Oner AF, Dillon MA, Swale RE, Wall KM, et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci U S A. 2008;105:5986–91.
Chen Z, Wang J, Bao L, Guo L, Zhang W, Xue Y, et al. Human monoclonal antibodies targeting the haemagglutinin glycoprotein can neutralize H7N9 influenza virus. Nat Commun. 2015;6.
Wang J, Chen Z, Bao L, Zhang W, Xue Y, Pang X, et al. Characterization of Two Human Monoclonal Antibodies Neutralizing Influenza A H7N9 Viruses. J Virol. 2015.
Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, Palser AL, Ong SH, et al. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. mBio. 2014;5.
Tang XC, Agnihothram SS, Jiao Y, Stanhope J, Graham RL, Peterson EC, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A. 2014;111:E2018–E26.
Lim CC, Woo PCY, Lim TS. Development of a Phage Display Panning Strategy Utilizing Crude Antigens: Isolation of MERS-CoV Nucleoprotein human antibodies. Sci Rep. 2019;9:6088.
Walker LM, Burton DR. Passive immunotherapy of viral infections: 'super-antibodies' enter the fray. Nat Rev Immunol. 2018;18:297–308.
Kennedy PJ, Oliveira C, Granja PL, Sarmento B. Monoclonal antibodies: technologies for early discovery and engineering. Crit Rev Biotechnol. 2018;38:394–408.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45.
Lindsley CW. Predictions and Statistics for the Best-Selling Drugs Globally and in the United States in 2018 and a Look Forward to 2024 Projections. ACS Chem Neurosci. 2019;10:1115.
Edwards BM, Barash SC, Main SH, Choi GH, Minter R, Ullrich S, et al. The remarkable flexibility of the human antibody repertoire; isolation of over one thousand different antibodies to a single protein, BLyS. J Mol Biol. 2003;334:103–18.
Sanz I, Yasothan U, Kirkpatrick P. Belimumab. Nat Rev Drug Discov. 2011;10:335.
Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends--is a paradigm change coming soon? Methods Mol Biol. 2009;525:1–27 xiii.
Diaz-Serrano A, Sanchez-Torre A, Paz-Ares L. Necitumumab for the treatment of advanced non-small-cell lung cancer. Future Oncol. 2019;15:705–16.
Arrieta O, Zatarain-Barron ZL, Cardona AF, Carmona A, Lopez-Mejia M. Ramucirumab in the treatment of non-small cell lung cancer. Expert Opin Drug Saf. 2017;16:637–44.
Aprile G, Ferrari L, Cremolini C, Bergamo F, Fontanella C, Battaglin F, et al. Ramucirumab for the treatment of gastric cancers, colorectal adenocarcinomas, and other gastrointestinal malignancies. Expert Rev Clin Pharmacol. 2016;9:877–85.
Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97:393–9.
Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, et al. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem. 2003;278:43496–507.
Frenzel A, Schirrmann T, Hust M. Phage display-derived human antibodies in clinical development and therapy. MAbs. 2016;8:1177–94.
Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res. 2015.
Rodriguez-Vida A, Bellmunt J. Avelumab for the treatment of urothelial cancer. Expert Rev Anticancer Ther. 2018;18:421–9.
Benson J, Cunningham M, Duchala C, Giles-Komar JM, Luo J, Rycyzyn MA, et al.; Janssen Biotech Inc., assignee. Anti-IL-23 antibodies, compositions, methods and uses. US patent US7807414B2. 2009.
Benson J, Carton J, Cunningham M, Orlovsky YI, Rauchenberger R and Sweet R; Janssen Biotech Inc., assignee. Anti-IL-23 antibody compositions. US patent US9714287B2. 2016.
Machado A, Torres T. Guselkumab for the Treatment of Psoriasis. Biodrugs. 2018;32:119–28.
Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119:267–74.
Cicardi M, Banerji A, Bracho F, Malbrán A, Rosenkranz B, Riedl M, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363:532–41.
Busse PJ, Farkas H, Banerji A, Lumry WR, Longhurst HJ, Sexton DJ, et al. Lanadelumab for the Prophylactic Treatment of Hereditary Angioedema with C1 Inhibitor Deficiency: A Review of Preclinical and Phase I Studies. BioDrugs. 2019;33:33–43.
Xu C, Sui J, Tao H, Zhu Q, Marasco WA. Human anti-CXCR4 antibodies undergo VH replacement, exhibit functional V-region sulfation, and define CXCR4 antigenic heterogeneity. J Immunol. 2007;179:2408–18.
Sheehan J and Marasco WA. Phage and Yeast Display. Microbiol Spectr. 2015;3:Aid-0028-2014.
Bradbury AR, Marks JD. Antibodies from phage antibody libraries. J Immunol Methods. 2004;290:29–49.
Jones AR, Stutz CC, Zhou Y, Marks JD, Shusta EV. Identifying blood-brain-barrier selective single-chain antibody fragments. Biotechnol J. 2014;9:664–74.
Bruggemann M, Osborn MJ, Ma B, Hayre J, Avis S, Lundstrom B, et al. Human antibody production in transgenic animals. Arch Immunol Ther Exp (Warsz). 2015;63:101–8.
Osborn MJ, Ma B, Avis S, Binnie A, Dilley J, Yang X, et al. High-affinity IgG antibodies develop naturally in Ig-knockout rats carrying germline human IgH/Igkappa/Iglambda loci bearing the rat CH region. J Immunol. 2013;190:1481–90.
Lee EC, Liang Q, Ali H, Bayliss L, Beasley A, Bloomfield-Gerdes T, et al. Complete humanization of the mouse immunoglobulin loci enables efficient therapeutic antibody discovery. Nat Biotechnol. 2014;32:356–63.
Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111:5153–8.
Alt FW, Keith Blackwell T, Yancopoulos GD. Immunoglobulin genes in transgenic mice. Trends Genet. 1985;1:231–6.
Bruggemann M, Caskey HM, Teale C, Waldmann H, Williams GT, Surani MA, et al. A repertoire of monoclonal antibodies with human heavy chains from transgenic mice. Proc Natl Acad Sci U S A. 1989;86:6709–13.
Taylor LD, Carmack CE, Schramm SR, Mashayekh R, Higgins KM, Kuo CC, et al. A transgenic mouse that expresses a diversity of human sequence heavy and light chain immunoglobulins. Nucleic Acids Res. 1992;20:6287–95.
Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–56.
Chen J, Trounstine M, Kurahara C, Young F, Kuo CC, Xu Y, et al. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993;12:821–30.
Davies NP, Rosewell IR, Richardson JC, Cook GP, Neuberger MS, Brownstein BH, et al. Creation of mice expressing human antibody light chains by introduction of a yeast artificial chromosome containing the core region of the human immunoglobulin kappa locus. Biotechnology (N Y). 1993;11:911–4.
Choi TK, Hollenbach PW, Pearson BE, Ueda RM, Weddell GN, Kurahara CG, et al. Transgenic mice containing a human heavy chain immunoglobulin gene fragment cloned in a yeast artificial chromosome. Nat Genet. 1993;4:117–23.
Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol. 2007;25:1134–43.
Hoffman W, Lakkis FG, Chalasani G. B Cells, Antibodies, and More. Clin J Am Soc Nephrol. 2016;11:137–54.
Diaz M, Casali P. Somatic immunoglobulin hypermutation. Curr Opin Immunol. 2002;14:235–40.
Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204.
Tyagi P. Recent results and ongoing trials with panitumumab (ABX-EGF), a fully human anti-epidermal growth factor receptor antibody, in metastatic colorectal cancer. Clin Colorectal Cancer. 2005;5:21–3.
Den Broeder, Alfons, et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002;29(11):2288–98.
Tyagi, Preeta, Chu E, Jain VK. Recent results and ongoing trials with panitumumab (ABXEGF), a fully human anti–epidermal growth factor receptor antibody, in metastatic colorectal cancer. Clin Colorectal Cancer. 2005;5(1):21–23.
Ding, Changhai. Belimumab, an anti-BLyS human monoclonal antibody for potential treatment of inflammatory autoimmune diseases. Expert Opin Biol Ther. 2008;8(11):1805–14.
Krupitskaya, Yelena, Wakelee HA. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs. 2009;10(6):597–605.
Kuenen, Bart, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res. 2010;16(6):1915–23.
McDermott, David F, et al. Atezolizumab, an anti–programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34(8):833–42.
Boyerinas, Benjamin, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti–PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immun Res. 2015;3(10):1148–57.
Sofen, Howard, et al. Guselkumab (an IL-23–specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–40.
Al-Salama, Zaina T. Emapalumab: first global approval. Drugs. 2019;79(1):99–103.
Kreitman, Robert J, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32(8):1768.
Berardi R, Onofri A, Pistelli M, Maccaroni E, Scartozzi M, Pierantoni C, et al. Panitumumab: the evidence for its use in the treatment of metastatic colorectal cancer. Core Evid. 2010;5:61–76.
Wong IT, Shojania K, Dutz J, Tsao NW. Clinical and economic review of secukinumab for moderate-to-severe plaque psoriasis. Expert Rev Pharmacoecon Outcomes Res. 2016;16:153–66.
Gibney GT, Hamid O, Lutzky J, Olszanski AJ, Mitchell TC, Gajewski TF, et al. Phase 1/2 study of epacadostat in combination with ipilimumab in patients with unresectable or metastatic melanoma. J Immunother Cancer. 2019;7:80.
Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015;11:1307–26.
Adedokun OJ, Xu Z, Gasink C, Jacobstein D, Szapary P, Johanns J, et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients With Crohn's Disease. Gastroenterology. 2018;154:1660–71.
Scott CT. Mice with a human touch. Nat Biotechnol. 2007;25:1075–7.
Legouffe E, Liautard J, Gaillard JP, Rossi JF, Wijdenes J, Bataille R, et al. Human anti-mouse antibody response to the injection of murine monoclonal antibodies against IL-6. Clin Exp Immunol. 1994;98:323–9.
Aman P, Ehlin-Henriksson B, Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984;159:208–20.
Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–5.
Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85:9998–10009.
Kuppers R, Zhao M, Hansmann ML, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993;12:4955–67.
Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4:372–84.
Obiakor H, Sehgal D, Dasso JF, Bonner RF, Malekafzali A, Mage RG. A comparison of hydraulic and laser capture microdissection methods for collection of single B cells, PCR, and sequencing of antibody VDJ. Anal Biochem. 2002;306:55–62.
Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–24.
Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7.
Lagerkvist AC, Furebring C, Borrebaeck CA. Single, antigen-specific B cells used to generate Fab fragments using CD40-mediated amplification or direct PCR cloning. Biotechniques. 1995;18:862–9.
Battye FL, Light A, Tarlinton DM. Single cell sorting and cloning. J Immunol Methods. 2000;243:25–32.
Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, Cutrell E, et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343:65–7.
Herzenberg LA, Parks D, Sahaf B, Perez O, Roederer M, Herzenberg LA. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin Chem. 2002;48:1819–27.
Lundkvist A, Horling J, Athlin L, Rosen A, Niklasson B. Neutralizing human monoclonal antibodies against Puumala virus, causative agent of nephropathia epidemica: a novel method using antigen-coated magnetic beads for specific B cell isolation. J Gen Virol. 1993;74(Pt 7):1303–10.
Correa I, Ilieva KM, Crescioli S, Lombardi S, Figini M, Cheung A, et al. Evaluation of Antigen-Conjugated Fluorescent Beads to Identify Antigen-Specific B Cells. Front Immunol. 2018;9:493.
Weitkamp JH, Kallewaard N, Kusuhara K, Feigelstock D, Feng N, Greenberg HB, et al. Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J Immunol Methods. 2003;275:223–37.
Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–61.
Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–40.
Smith SA, de Alwis AR, Kose N, Jadi RS, de Silva AM, Crowe JE Jr. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J Virol. 2014;88:12233–41.
Woda M, Friberg H, Currier JR, Srikiatkhachorn A, Macareo LR, Green S, et al. Dynamics of Dengue Virus (DENV)-Specific B Cells in the Response to DENV Serotype 1 Infections, Using Flow Cytometry With Labeled Virions. J Infect Dis. 2016;214:1001–9.
Coronella JA, Telleman P, Truong TD, Ylera F, Junghans RP. Amplification of IgG VH and VL (Fab) from single human plasma cells and B cells. Nucleic Acids Res. 2000;28:E85.
Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71.
Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, Kinoshita K, et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat Med. 2009;15:1088–92.
Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat Biotechnol. 2006;24:703–7.
Ogunniyi AO, Story CM, Papa E, Guillen E, Love JC. Screening individual hybridomas by microengraving to discover monoclonal antibodies. Nat Protoc. 2009;4:767–82.
Tajiri K, Kishi H, Tokimitsu Y, Kondo S, Ozawa T, Kinoshita K, et al. Cell-microarray analysis of antigen-specific B-cells: single cell analysis of antigen receptor expression and specificity. Cytometry A. 2007;71:961–7.
Tokimitsu Y, Kishi H, Kondo S, Honda R, Tajiri K, Motoki K, et al. Single lymphocyte analysis with a microwell array chip. Cytometry A. 2007;71:1003–10.
Smith K, Crowe SR, Garman L, Guthridge CJ, Muther JJ, McKee E, et al. Human monoclonal antibodies generated following vaccination with AVA provide neutralization by blocking furin cleavage but not by preventing oligomerization. Vaccine. 2012;30:4276–83.
Chi X, Li J, Liu W, Wang X, Yin K, Liu J, et al. Generation and Characterization of Human Monoclonal Antibodies Targeting Anthrax Protective Antigen following Vaccination with a Recombinant Protective Antigen Vaccine. Clin Vacc Immunol: CVI. 2015;22:553–60.
Rudkin FM, Raziunaite I, Workman H, Essono S, Belmonte R, MacCallum DM, et al. Single human B cell-derived monoclonal anti-Candida antibodies enhance phagocytosis and protect against disseminated candidiasis. Nat Commun. 2018;9:5288.
Cox KS, Tang A, Chen Z, Horton MS, Yan H, Wang XM, et al. Rapid isolation of dengue-neutralizing antibodies from single cell-sorted human antigen-specific memory B-cell cultures. MAbs. 2016;8:129–40.
Iizuka A, Komiyama M, Tai S, Oshita C, Kurusu A, Kume A, et al. Identification of cytomegalovirus (CMV)pp65 antigen-specific human monoclonal antibodies using single B cell-based antibody gene cloning from melanoma patients. Immunol Lett. 2011;135:64–73.
Tian C, Luskin GK, Dischert KM, Higginbotham JN, Shepherd BE, Crowe JE Jr. Immunodominance of the VH1–46 antibody gene segment in the primary repertoire of human rotavirus-specific B cells is reduced in the memory compartment through somatic mutation of nondominant clones. J Immunol. 2008;180:3279–88.
Di Niro R, Mesin L, Raki M, Zheng NY, Lund-Johansen F, Lundin KE, et al. Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa. J Immunol. 2010;185:5377–83.
Bailey MJ, Duehr J, Dulin H, Broecker F, Brown JA, Arumemi FO, et al. Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model. Nat Commun. 2018;9:4560.
Bushey RT, Moody MA, Nicely NL, Haynes BF, Alam SM, Keir ST, et al. A Therapeutic Antibody for Cancer, Derived from Single Human B Cells. Cell Rep. 2016;15:1505–13.
Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351:1339–42.
Rijal P, Elias SC, Machado SR, Xiao J, Schimanski L, O'Dowd V, et al. Therapeutic Monoclonal Antibodies for Ebola Virus Infection Derived from Vaccinated Humans. Cell Rep. 2019;27:172–86 e7.
Bornholdt ZA, Turner HL, Murin CD, Li W, Sok D, Souders CA, et al. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science. 2016;351:1078–83.
Awi NJ, Teow SY. Antibody-Mediated Therapy against HIV/AIDS: Where Are We Standing Now? J Pathog. 2018;2018:8724549.
Nogales A, Piepenbrink MS, Wang J, Ortega S, Basu M, Fucile CF, et al. A Highly Potent and Broadly Neutralizing H1 Influenza-Specific Human Monoclonal Antibody. Sci Rep. 2018;8:4374.
Fu Y, Zhang Z, Sheehan J, Avnir Y, Ridenour C, Sachnik T, et al. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat Commun. 2016;7:12780.
Nakamura G, Chai N, Park S, Chiang N, Lin Z, Chiu H, et al. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe. 2013;14:93–103.
Chai N, Swem LR, Park S, Nakamura G, Chiang N, Estevez A, et al. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat Commun. 2017;8:14234.
Gilman MS, Castellanos CA, Chen M, Ngwuta JO, Goodwin E, Moin SM, et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol. 2016;1.
Goodwin E, Gilman MSA, Wrapp D, Chen M, Ngwuta JO, Moin SM, et al. Infants Infected with Respiratory Syncytial Virus Generate Potent Neutralizing Antibodies that Lack Somatic Hypermutation. Immunity. 2018;48:339–49 e5.
Doria-Rose NA, Joyce MG. Strategies to guide the antibody affinity maturation process. Curr Opin Virol. 2015;11:137–47.
Eisen HN. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014;2:381–92.
Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–8.
Makabe K, Nakanishi T, Tsumoto K, Tanaka Y, Kondo H, Umetsu M, et al. Thermodynamic consequences of mutations in vernier zone residues of a humanized anti-human epidermal growth factor receptor murine antibody, 528. J Biol Chem. 2008;283:1156–66.
Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–16.
Ho M, Kreitman RJ, Onda M, Pastan I. In vitro antibody evolution targeting germline hot spots to increase activity of an anti-CD22 immunotoxin. J Biol Chem. 2005;280:607–17.
Thie H, Voedisch B, Dübel S, Hust M, Schirrmann T. Affinity Maturation by Phage Display. In: Dimitrov AS, editor. Therapeutic Antibodies. Totowa: Humana Press; 2009. p. 309–22.
Steinwand M, Droste P, Frenzel A, Hust M, Dübel S, Schirrmann T. The influence of antibody fragment format on phage display based affinity maturation of IgG. MAbs. 2013;6:204–18.
Low NM, Holliger PH, Winter G. Mimicking somatic hypermutation: affinity maturation of antibodies displayed on bacteriophage using a bacterial mutator strain. J Mol Biol. 1996;260:359–68.
Marks JD. Antibody affinity maturation by chain shuffling. Methods Mol Biol. 2004;248:327–43.
Nielsen UB, Marks JD. Affinity maturation of phage antibodies. In: Clackson T, Lowman HB, editors. Phage Display: A Practical Approach. Oxford: Oxford University Press; 2004. p. 360.
Saunders KO. Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Front Immunol. 2019;10:1296.
Kelley RF, Meng YG. Methods to engineer and identify IgG1 variants with improved FcRn binding or effector function. Methods Mol Biol. 2012;901:277–93.
Liu Z, Gunasekaran K, Wang W, Razinkov V, Sekirov L, Leng E, et al. Asymmetrical Fc engineering greatly enhances antibody-dependent cellular cytotoxicity (ADCC) effector function and stability of the modified antibodies. J Biol Chem. 2014;289:3571–90.
Monnet C, Jorieux S, Souyris N, Zaki O, Jacquet A, Fournier N, et al. Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody. MAbs. 2014;6:422–36.
Mimura Y, Katoh T, Saldova R, O'Flaherty R, Izumi T, Mimura-Kimura Y, et al. Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy. Protein Cell. 2018;9:47–62.
Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A. 2017;114:3485–90.
Chen CL, Hsu JC, Lin CW, Wang CH, Tsai MH, Wu CY, et al. Crystal Structure of a Homogeneous IgG-Fc Glycoform with the N-Glycan Designed to Maximize the Antibody Dependent Cellular Cytotoxicity. ACS Chem Biol. 2017;12:1335–45.
Lin CW, Tsai MH, Li ST, Tsai TI, Chu KC, Liu YC, et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc Natl Acad Sci U S A. 2015;112:10611–6.
Neri D. Antibody-Cytokine Fusions: Versatile Products for the Modulation of Anticancer Immunity. Cancer Immunol Res. 2019;7:348–54.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017;16:315.
Larson SM, Carrasquillo JA, Cheung N-KV, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347.
Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019.
Ohradanova-Repic A, Nogueira E, Hartl I, Gomes AC, Preto A, Steinhuber E, et al. Fab antibody fragment-functionalized liposomes for specific targeting of antigen-positive cells. Nanomedicine. 2018;14:123–30.
Lu R-M, Chang Y-L, Chen M-S, Wu H-C. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials. 2011;32:3265–74.
June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379:64–73.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45–56.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378:449–59.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48.
Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2017;15:31.
Chen IC, Chiu YK, Yu CM, Lee CC, Tung CP, Tsou YL, et al. High throughput discovery of influenza virus neutralizing antibodies from phage-displayed synthetic antibody libraries. Sci Rep. 2017;7:14455.
Barreto K, Maruthachalam BV, Hill W, Hogan D, Sutherland AR, Kusalik A, et al. Next-generation sequencing-guided identification and reconstruction of antibody CDR combinations from phage selection outputs. Nucleic Acids Res. 2019.
Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2017;18:35.
Georgiou G, Ippolito GC, Beausang J, Busse CE, Wardemann H, Quake SR. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat Biotechnol. 2014;32:158.
Research and Markets. Global and China Monoclonal Antibody Industry Report, 2019–2025. Global, China 2019, April. 180 p.
Acknowledgements
Not applicable.
Funding
This work was supported by Academia Sinica and Ministry of Science and Technology [106-0210-01-15-02] and [107-0210-01-19-01], and the Program for Translational Innovation of Biopharmaceutical Development - Technology Supporting Platform Axis and [107-0210-01-19-04], Taiwan (to H.-C. Wu).
Author information
Authors and Affiliations
Contributions
R-M L, Y-C H, I-J L, C-C L, H-Z T, H-J L, and H-C W wrote the manuscript. R-M L, C-C L, H-Z T, and H-C W designed and illustrated Table. R-M L, Y-C H, I-J L, C-C L, H-Z T, H-J L, and H-C W designed and illustrated figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lu, RM., Hwang, YC., Liu, IJ. et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27, 1 (2020). https://doi.org/10.1186/s12929-019-0592-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-019-0592-z